Activity of the β-Lactamase Inhibitor LN-1-255 against Carbapenem-Hydrolyzing Class D β-Lactamases from Acinetobacter baumannii
- PMID: 28807908
- PMCID: PMC5655052
- DOI: 10.1128/AAC.01172-17
Activity of the β-Lactamase Inhibitor LN-1-255 against Carbapenem-Hydrolyzing Class D β-Lactamases from Acinetobacter baumannii
Abstract
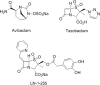
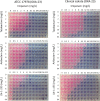
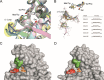
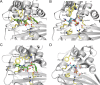
The number of infections caused by Gram-negative pathogens carrying carbapenemases is increasing, and the group of carbapenem-hydrolyzing class D β-lactamases (CHDLs) is especially problematic. Several clinically important CHDLs have been identified in Acinetobacter baumannii, including OXA-23, OXA-24/40, OXA-58, OXA-143, OXA-235, and the chromosomally encoded OXA-51. The selection and dissemination of carbapenem-resistant A. baumannii strains constitutes a serious global threat. Carbapenems have been successfully utilized as last-resort antibiotics for the treatment of multidrug-resistant A. baumannii infections. However, the spread of OXA carbapenemases is compromising the continued use of these antimicrobials. In response to this clinical issue, it is necessary and urgent to design and develop new specific inhibitors with efficacy against these enzymes. The aim of this work was to characterize the inhibitory activity of LN-1-255 (a 6-alkylidene-2-substituted penicillin sulfone) and compare it to that of two established inhibitors (avibactam and tazobactam) against the most relevant enzymes of each group of class D carbapenemases in A. baumannii The β-lactamase inhibitor LN-1-255 demonstrated excellent microbiological synergy and inhibition kinetics parameters against all tested CHDLs and a significantly higher activity than tazobactam and avibactam. A combination of carbapenems and LN-1-255 was effective against A. baumannii class D carbapenemases. Docking assays confirmed the affinity of LN-1-255 for the active site of these enzymes. LN-1-255 represents a potential new β-lactamase inhibitor that may have a significant role in eradicating infections caused by A. baumannii isolates carrying CHDLs.
Keywords: Acinetobacter baumannii; antimicrobial resistance; carbapenem-hydrolyzing class D β-lactamases; β-lactamase inhibitors.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, Leblebicioglu H, Abu Khader I, Miranda Novales MG, Berba R, Ramírez Wong FM, Barkat A, Pino OR, Dueñas L, Mitrev Z, Bijie H, Gurskis V, Kanj SS, Mapp T, Hidalgo RF, Ben Jaballah N, Raka L, Gikas A, Ahmed A, Thu TA, Guzmán Siritt ME, INICC Members 2010. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control 38:95–104.e2. doi: 10.1016/j.ajic.2009.12.004. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

