Systematic Review and Meta-analyses of the Effect of Chemotherapy on Pulmonary Mycobacterium abscessus Outcomes and Disease Recurrence
- PMID: 28807911
- PMCID: PMC5655093
- DOI: 10.1128/AAC.01206-17
Systematic Review and Meta-analyses of the Effect of Chemotherapy on Pulmonary Mycobacterium abscessus Outcomes and Disease Recurrence
Abstract
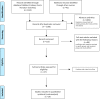
In pharmacokinetic/pharmacodynamic models of pulmonary Mycobacterium abscessus complex, the recommended macrolide-containing combination therapy has poor kill rates. However, clinical outcomes are unknown. We searched the literature for studies published between 1990 and 2017 that reported microbial outcomes in patients treated for pulmonary M. abscessus disease. A good outcome was defined as sustained sputum culture conversion (SSCC) without relapse. Random effects models were used to pool studies and estimate proportions of patients with good outcomes. Odds ratios (OR) and 95% confidence intervals (CI) were computed. Sensitivity analyses and metaregression were used to assess the robustness of findings. In 19 studies of 1,533 patients, combination therapy was administered to 508 patients with M. abscessus subsp. abscessus, 204 with M. abscessus subsp. massiliense, and 301 with M. abscessus with no subspecies specified. Macrolide-containing regimens achieved SSCC in only 77/233 (34%) new M. abscessus subsp. abscessus patients versus 117/141 (54%) M. abscessus subsp. massiliense patients (OR, 0.108 [95% CI, 0.066 to 0.181]). In refractory disease, SSCC was achieved in 20% (95% CI, 7 to 36%) of patients, which was not significantly different across subspecies. The estimated recurrent rates per month were 1.835% (range, 1.667 to 3.196%) for M. abscessus subsp. abscessus versus 0.683% (range, 0.229 to 1.136%) for M. abscessus subsp. massiliense (OR, 6.189 [95% CI, 2.896 to 13.650]). The proportion of patients with good outcomes was 52/223 (23%) with M. abscessus subsp. abscessus versus 118/141 (84%) with M. abscessus subsp. massiliense disease (OR, 0.059 [95% CI, 0.034 to 0.101]). M. abscessus subsp. abscessus pulmonary disease outcomes with the currently recommended regimens are atrocious, with outcomes similar to those for extensively drug-resistant tuberculosis. Therapeutically, the concept of nontuberculous mycobacteria is misguided. There is an urgent need to craft entirely new treatment regimens.
Keywords: Mycobacterium abscessus; hollow-fiber model; macrolides; medical outcomes; pulmonary infection.
Copyright © 2017 Pasipanodya et al.
Figures



Similar articles
-
Clinical Characteristics and Treatment Outcomes of Patients with Acquired Macrolide-Resistant Mycobacterium abscessus Lung Disease.Antimicrob Agents Chemother. 2017 Sep 22;61(10):e01146-17. doi: 10.1128/AAC.01146-17. Print 2017 Oct. Antimicrob Agents Chemother. 2017. PMID: 28739795 Free PMC article.
-
Association between sequevar and antibiotic treatment outcome in patients with Mycobacterium abscessus complex infections in Japan.J Med Microbiol. 2018 Jan;67(1):74-82. doi: 10.1099/jmm.0.000661. Epub 2017 Dec 11. J Med Microbiol. 2018. PMID: 29227218
-
Successful antibiotic treatment of pulmonary disease caused by Mycobacterium abscessus subsp. abscessus with C-to-T mutation at position 19 in erm(41) gene: case report.BMC Infect Dis. 2016 May 17;16:207. doi: 10.1186/s12879-016-1554-7. BMC Infect Dis. 2016. PMID: 27188784 Free PMC article.
-
Treatment of Mycobacterium abscessus Pulmonary Disease.Chest. 2022 Jan;161(1):64-75. doi: 10.1016/j.chest.2021.07.035. Epub 2021 Jul 24. Chest. 2022. PMID: 34314673 Review.
-
[A pharmacologic approach to treatment of Mycobacterium abscessus pulmonary disease].Rev Mal Respir. 2024 Jan;41(1):29-42. doi: 10.1016/j.rmr.2023.10.010. Epub 2023 Nov 27. Rev Mal Respir. 2024. PMID: 38016833 Review. French.
Cited by
-
Mycobacterium abscessus complex: A Review of Recent Developments in an Emerging Pathogen.Front Cell Infect Microbiol. 2021 Apr 26;11:659997. doi: 10.3389/fcimb.2021.659997. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33981630 Free PMC article. Review.
-
Population Pharmacokinetic Analysis of Bedaquiline-Clarithromycin for Dose Selection Against Pulmonary Nontuberculous Mycobacteria Based on a Phase 1, Randomized, Pharmacokinetic Study.J Clin Pharmacol. 2021 Oct;61(10):1344-1355. doi: 10.1002/jcph.1887. Epub 2021 Jun 12. J Clin Pharmacol. 2021. PMID: 33991350 Free PMC article. Clinical Trial.
-
In Vitro Synergistic Effects of Omadacycline with Other Antimicrobial Agents against Mycobacterium abscessus.Antimicrob Agents Chemother. 2023 Jun 15;67(6):e0157922. doi: 10.1128/aac.01579-22. Epub 2023 May 8. Antimicrob Agents Chemother. 2023. PMID: 37154742 Free PMC article.
-
Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline.Eur Respir J. 2020 Jul 7;56(1):2000535. doi: 10.1183/13993003.00535-2020. Print 2020 Jul. Eur Respir J. 2020. PMID: 32636299 Free PMC article.
-
Medical Costs of Nontuberculous Mycobacterial Pulmonary Disease, South Korea, 2015-2019.Emerg Infect Dis. 2024 Sep;30(9):1841-1849. doi: 10.3201/eid3009.231448. Emerg Infect Dis. 2024. PMID: 39173659 Free PMC article.
References
-
- Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi:10.1016/S0140-6736(13)60632-7. - DOI - PMC - PubMed
-
- Baker AW, Lewis SS, Alexander BD, Chen LF, Wallace RJ Jr, Brown-Elliott BA, Isaacs PJ, Pickett LC, Patel CB, Smith PK, Reynolds JM, Engel J, Wolfe CR, Milano CA, Schroder JN, Davis RD, Hartwig MG, Stout JE, Strittholt N, Maziarz EK, Saullo JH, Hazen KC, Walczak RJ Jr, Vasireddy R, Vasireddy S, McKnight CM, Anderson DJ, Sexton DJ. 2017. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 64:902–911. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

