Relationships of Vancomycin Pharmacokinetics to Body Size and Composition Using a Novel Pharmacomorphomic Approach Based on Medical Imaging
- PMID: 28807918
- PMCID: PMC5655058
- DOI: 10.1128/AAC.01402-17
Relationships of Vancomycin Pharmacokinetics to Body Size and Composition Using a Novel Pharmacomorphomic Approach Based on Medical Imaging
Abstract
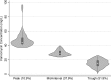
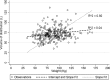
Antibiotics such as vancomycin are empirically dosed on the basis of body weight, which may not be optimal across the expanding adult body size distribution. Our aim was to compare the relationships between morphomic parameters generated from computed tomography images to conventional body size metrics as predictors of vancomycin pharmacokinetics (PK). This single-center retrospective study included 300 patients with 1,622 vancomycin concentration (52% trough) measurements. Bayesian estimation was used to compute individual vancomycin volume of distribution of the central compartment (Vc) and clearance (CL). Approximately 45% of patients were obese with an overall median (5th, 95th percentile) weight and body mass index of 87.2 (54.7, 123) kg and 28.8 (18.9, 43.7) kg/m2, respectively. Morphomic parameters of body size such as body depth, total body area, and torso volume of the twelfth thoracic through fourth lumbar vertebrae (T12 to L4) correlated with Vc. The relationship of vancomycin Vc was poorly predicted by body size but was stronger with T12-to-L4 torso volume (coefficient of determination [R2] = 0.11) than weight (R2 = 0.04). No relationships between vancomycin CL and traditional body size metrics could be discerned; however, relationships with skeletal muscle volume and total psoas area were found. Vancomycin CL independently correlated with total psoas area and inversely correlated with age. Thus, vancomycin CL was significantly related to total psoas area over age (R2 = 0.23, P < 0.0001). This proof-of-concept study suggests a potential role for translation of radiographic information into parameters predictive of drug pharmacokinetics. Prediction of individual antimicrobial pharmacokinetic parameters using analytic morphomics has the potential to improve antimicrobial dose selection and outcomes of obese patients.
Keywords: antimicrobial safety; drug dosing; morphomics; muscle; precision medicine; psoas; safety.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) 2011. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377:557–567. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

