CD177 modulates human neutrophil migration through activation-mediated integrin and chemoreceptor regulation
- PMID: 28807980
- PMCID: PMC5680608
- DOI: 10.1182/blood-2017-03-768507
CD177 modulates human neutrophil migration through activation-mediated integrin and chemoreceptor regulation
Abstract
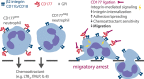
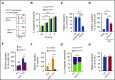
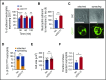
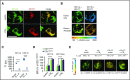
CD177 is a glycosylphosphatidylinositol (GPI)-anchored protein expressed by a variable proportion of human neutrophils that mediates surface expression of the antineutrophil cytoplasmic antibody antigen proteinase 3. CD177 associates with β2 integrins and recognizes platelet endothelial cell adhesion molecule 1 (PECAM-1), suggesting a role in neutrophil migration. However, CD177pos neutrophils exhibit no clear migratory advantage in vivo, despite interruption of in vitro transendothelial migration by CD177 ligation. We sought to understand this paradox. Using a PECAM-1-independent transwell system, we found that CD177pos and CD177neg neutrophils migrated comparably. CD177 ligation selectively impaired migration of CD177pos neutrophils, an effect mediated through immobilization and cellular spreading on the transwell membrane. Correspondingly, CD177 ligation enhanced its interaction with β2 integrins, as revealed by fluorescence lifetime imaging microscopy, leading to integrin-mediated phosphorylation of Src and extracellular signal-regulated kinase (ERK). CD177-driven cell activation enhanced surface β2 integrin expression and affinity, impaired internalization of integrin attachments, and resulted in ERK-mediated attenuation of chemokine signaling. We conclude that CD177 signals in a β2 integrin-dependent manner to orchestrate a set of activation-mediated mechanisms that impair human neutrophil migration.
© 2017 by The American Society of Hematology.
Figures


References
-
- Stroncek DF, Shankar RA, Noren PA, Herr GP, Clement LT. Analysis of the expression of NB1 antigen using two monoclonal antibodies. Transfusion. 1996;36(2):168-174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

