Two transmembrane dimers of the bovine papillomavirus E5 oncoprotein clamp the PDGF β receptor in an active dimeric conformation
- PMID: 28808001
- PMCID: PMC5584431
- DOI: 10.1073/pnas.1705622114
Two transmembrane dimers of the bovine papillomavirus E5 oncoprotein clamp the PDGF β receptor in an active dimeric conformation
Abstract
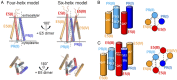
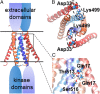
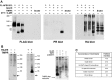
The dimeric 44-residue E5 protein of bovine papillomavirus is the smallest known naturally occurring oncoprotein. This transmembrane protein binds to the transmembrane domain (TMD) of the platelet-derived growth factor β receptor (PDGFβR), causing dimerization and activation of the receptor. Here, we use Rosetta membrane modeling and all-atom molecular dynamics simulations in a membrane environment to develop a chemically detailed model of the E5 protein/PDGFβR complex. In this model, an active dimer of the PDGFβR TMD is sandwiched between two dimers of the E5 protein. Biochemical experiments showed that the major PDGFβR TMD complex in mouse cells contains two E5 dimers and that binding the PDGFβR TMD to the E5 protein is necessary and sufficient to recruit both E5 dimers into the complex. These results demonstrate how E5 binding induces receptor dimerization and define a molecular mechanism of receptor activation based on specific interactions between TMDs.
Keywords: BPV; blue native gel electrophoresis; oncogene; transmembrane protein complex; traptamer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
The bovine papillomavirus E5 protein and the PDGF beta receptor: it takes two to tango.Virology. 2009 Feb 20;384(2):345-51. doi: 10.1016/j.virol.2008.09.033. Epub 2008 Nov 6. Virology. 2009. PMID: 18990418 Free PMC article. Review.
-
Multiple transmembrane amino acid requirements suggest a highly specific interaction between the bovine papillomavirus E5 oncoprotein and the platelet-derived growth factor beta receptor.J Virol. 2002 Aug;76(16):7976-86. doi: 10.1128/jvi.76.16.7976-7986.2002. J Virol. 2002. PMID: 12134002 Free PMC article.
-
Molecular examination of the transmembrane requirements of the platelet-derived growth factor beta receptor for a productive interaction with the bovine papillomavirus E5 oncoprotein.J Biol Chem. 2002 Dec 6;277(49):47149-59. doi: 10.1074/jbc.M209582200. Epub 2002 Sep 25. J Biol Chem. 2002. PMID: 12351659
-
Role of glutamine 17 of the bovine papillomavirus E5 protein in platelet-derived growth factor beta receptor activation and cell transformation.J Virol. 1998 Nov;72(11):8921-32. doi: 10.1128/JVI.72.11.8921-8932.1998. J Virol. 1998. PMID: 9765437 Free PMC article.
-
The platelet-derived growth factor beta receptor as a target of the bovine papillomavirus E5 protein.Cytokine Growth Factor Rev. 2000 Dec;11(4):283-93. doi: 10.1016/s1359-6101(00)00012-5. Cytokine Growth Factor Rev. 2000. PMID: 10959076 Review.
Cited by
-
De novo-designed transmembrane proteins bind and regulate a cytokine receptor.Nat Chem Biol. 2024 Jun;20(6):751-760. doi: 10.1038/s41589-024-01562-z. Epub 2024 Mar 13. Nat Chem Biol. 2024. PMID: 38480980 Free PMC article.
-
Molecular Detection and Quantification of Ovine Papillomavirus DNA in Equine Sarcoid.Transbound Emerg Dis. 2024 Feb 9;2024:6453158. doi: 10.1155/2024/6453158. eCollection 2024. Transbound Emerg Dis. 2024. PMID: 40303025 Free PMC article.
-
In silico molecular docking and dynamic simulation of anti-cholinesterase compounds from the extract of Catunaregam spinosa for possible treatment of Alzheimer's disease.Heliyon. 2024 Mar 20;10(7):e27880. doi: 10.1016/j.heliyon.2024.e27880. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38560123 Free PMC article.
-
Conformational Clamping by a Membrane Ligand Activates the EphA2 Receptor.J Mol Biol. 2021 Sep 3;433(18):167144. doi: 10.1016/j.jmb.2021.167144. Epub 2021 Jul 3. J Mol Biol. 2021. PMID: 34229012 Free PMC article.
-
Digital droplet PCR-based detection and quantification of ovine papillomavirus DNA from the vaginal virobiota of healthy mares.Sci Rep. 2025 Mar 22;15(1):9951. doi: 10.1038/s41598-025-94279-5. Sci Rep. 2025. PMID: 40121289 Free PMC article.
References
-
- Burkhardt A, Willingham M, Gay C, Jeang K-T, Schlegel R. The E5 oncoprotein of bovine papillomavirus is oriented asymmetrically in Golgi and plasma membranes. Virology. 1989;170:334–339. - PubMed
-
- Schlegel R, Wade-Glass M, Rabson MS, Yang Y-C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986;233:464–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

