Two transmembrane dimers of the bovine papillomavirus E5 oncoprotein clamp the PDGF β receptor in an active dimeric conformation
- PMID: 28808001
- PMCID: PMC5584431
- DOI: 10.1073/pnas.1705622114
Two transmembrane dimers of the bovine papillomavirus E5 oncoprotein clamp the PDGF β receptor in an active dimeric conformation
Abstract
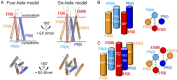
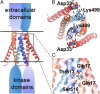
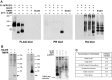
The dimeric 44-residue E5 protein of bovine papillomavirus is the smallest known naturally occurring oncoprotein. This transmembrane protein binds to the transmembrane domain (TMD) of the platelet-derived growth factor β receptor (PDGFβR), causing dimerization and activation of the receptor. Here, we use Rosetta membrane modeling and all-atom molecular dynamics simulations in a membrane environment to develop a chemically detailed model of the E5 protein/PDGFβR complex. In this model, an active dimer of the PDGFβR TMD is sandwiched between two dimers of the E5 protein. Biochemical experiments showed that the major PDGFβR TMD complex in mouse cells contains two E5 dimers and that binding the PDGFβR TMD to the E5 protein is necessary and sufficient to recruit both E5 dimers into the complex. These results demonstrate how E5 binding induces receptor dimerization and define a molecular mechanism of receptor activation based on specific interactions between TMDs.
Keywords: BPV; blue native gel electrophoresis; oncogene; transmembrane protein complex; traptamer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Burkhardt A, Willingham M, Gay C, Jeang K-T, Schlegel R. The E5 oncoprotein of bovine papillomavirus is oriented asymmetrically in Golgi and plasma membranes. Virology. 1989;170:334–339. - PubMed
-
- Schlegel R, Wade-Glass M, Rabson MS, Yang Y-C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986;233:464–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

