A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle
- PMID: 28808052
- PMCID: PMC5633120
- DOI: 10.1074/jbc.M117.776815
A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle
Abstract
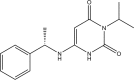
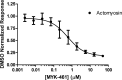
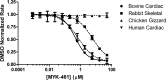
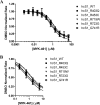
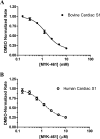
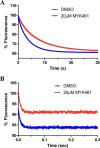
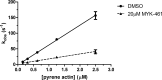
Mavacamten, formerly known as MYK-461 is a recently discovered novel small-molecule modulator of cardiac myosin that targets the underlying sarcomere hypercontractility of hypertrophic cardiomyopathy, one of the most prevalent heritable cardiovascular disorders. Studies on isolated cells and muscle fibers as well as intact animals have shown that mavacamten inhibits sarcomere force production, thereby reducing cardiac contractility. Initial mechanistic studies have suggested that mavacamten primarily reduces the steady-state ATPase activity by inhibiting the rate of phosphate release of β-cardiac myosin-S1, but the molecular mechanism of action of mavacamten has not been described. Here we used steady-state and presteady-state kinetic analyses to investigate the mechanism of action of mavacamten. Transient kinetic analyses revealed that mavacamten modulates multiple steps of the myosin chemomechanical cycle. In addition to decreasing the rate-limiting step of the cycle (phosphate release), mavacamten reduced the number of myosin-S1 heads that can interact with the actin thin filament during transition from the weakly to the strongly bound state without affecting the intrinsic rate. Mavacamten also decreased the rate of myosin binding to actin in the ADP-bound state and the ADP-release rate from myosin-S1 alone. We, therefore, conclude that mavacamten acts on multiple stages of the myosin chemomechanical cycle. Although the primary mechanism of mavacamten-mediated inhibition of cardiac myosin is the decrease of phosphate release from β-cardiac myosin-S1, a secondary mechanism decreases the number of actin-binding heads transitioning from the weakly to the strongly bound state, which occurs before phosphate release and may provide an additional method to modulate myosin function.
Keywords: MYK-461; cardiac hypertrophy; cardiomyopathy; myosin; presteady-state kinetics; sarcomere; small molecule.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
All authors are stockholders of MyoKardia, Inc
Figures

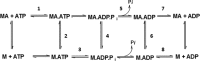

References
-
- Maron B. J., Gardin J. M., Flack J. M., Gidding S. S., Kurosaki T. T., and Bild D. E. (1995) Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. coronary artery risk development in (young) adults. Circulation 92, 785–789 - PubMed
-
- Wilson W. S., Criley J. M., and Ross R. S. (1967) Dynamics of left ventricular emptying in hypertrophic subaortic stenosis: A cinaengiographic and hemodynamic study. Am. Heart J. 73, 4–16 - PubMed
-
- Stewart S., Mason D. T., and Braunwald E. (1968) Impared rate of left ventricular filling in idiopathic hypertrophic subaortic stenosis and valvular aortic stenosis. Circulation 37, 8–14 - PubMed
-
- Ramaraj R. (2008) Hypertrophic cardiomyopathy: etiology, diagnosis, and treatment. Cardiol. Rev. 16, 172–180 - PubMed
-
- Frey N., Luedde M., and Katus H. A. (2011) Mechanisms of disease: hypertrophic cardiomyopathy. Nat. Rev. Cardiol. 9, 91–100 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

