Linchpin DNA-binding residues serve as go/no-go controls in the replication factor C-catalyzed clamp-loading mechanism
- PMID: 28808059
- PMCID: PMC5612119
- DOI: 10.1074/jbc.M117.798702
Linchpin DNA-binding residues serve as go/no-go controls in the replication factor C-catalyzed clamp-loading mechanism
Abstract
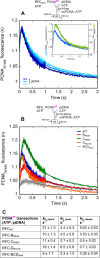
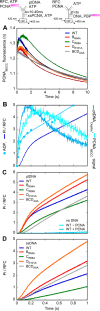
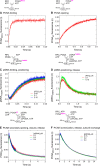
DNA polymerases depend on circular sliding clamps for processive replication. Clamps must be loaded onto primer-template DNA (ptDNA) by clamp loaders that open and close clamps around ptDNA in an ATP-fueled reaction. All clamp loaders share a core structure in which five subunits form a spiral chamber that binds the clamp at its base in a twisted open form and encloses ptDNA within, while binding and hydrolyzing ATP to topologically link the clamp and ptDNA. To understand how clamp loaders perform this complex task, here we focused on conserved arginines that might play a central coordinating role in the mechanism because they can alternately contact ptDNA or Walker B glutamate in the ATPase site and lie close to the clamp loader-clamp-binding interface. We mutated Arg-84, Arg-88, and Arg-101 in the ATPase-active B, C, and D subunits of Saccharomyces cerevisiae replication factor C (RFC) clamp loader, respectively, and assessed the impact on multiple transient events in the reaction: proliferating cell nuclear antigen (PCNA) clamp binding/opening/closure/release, ptDNA binding/release, and ATP hydrolysis/product release. The results show that these arginines relay critical information between the PCNA-binding, DNA-binding, and ATPase sites at all steps of the reaction, particularly at a checkpoint before RFC commits to ATP hydrolysis. Moreover, their actions are subunit-specific with RFC-C Arg-88 serving as an accelerator that enables rapid ATP hydrolysis upon contact with ptDNA and RFC-D Arg-101 serving as a brake that confers specificity for ptDNA as the correct substrate for loading PCNA.
Keywords: ATPase; DNA replication; clamp loader; pre-steady-state kinetics; proliferating cell nuclear antigen (PCNA); replication factor C (RFC).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

