The impact of IUGR on pancreatic islet development and β-cell function
- PMID: 28808079
- PMCID: PMC5808569
- DOI: 10.1530/JOE-17-0076
The impact of IUGR on pancreatic islet development and β-cell function
Abstract
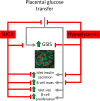
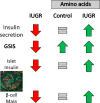
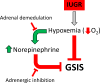
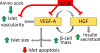
Placental insufficiency is a primary cause of intrauterine growth restriction (IUGR). IUGR increases the risk of developing type 2 diabetes mellitus (T2DM) throughout life, which indicates that insults from placental insufficiency impair β-cell development during the perinatal period because β-cells have a central role in the regulation of glucose tolerance. The severely IUGR fetal pancreas is characterized by smaller islets, less β-cells, and lower insulin secretion. Because of the important associations among impaired islet growth, β-cell dysfunction, impaired fetal growth, and the propensity for T2DM, significant progress has been made in understanding the pathophysiology of IUGR and programing events in the fetal endocrine pancreas. Animal models of IUGR replicate many of the observations in severe cases of human IUGR and allow us to refine our understanding of the pathophysiology of developmental and functional defects in islet from IUGR fetuses. Almost all models demonstrate a phenotype of progressive loss of β-cell mass and impaired β-cell function. This review will first provide evidence of impaired human islet development and β-cell function associated with IUGR and the impact on glucose homeostasis including the development of glucose intolerance and diabetes in adulthood. We then discuss evidence for the mechanisms regulating β-cell mass and insulin secretion in the IUGR fetus, including the role of hypoxia, catecholamines, nutrients, growth factors, and pancreatic vascularity. We focus on recent evidence from experimental interventions in established models of IUGR to understand better the pathophysiological mechanisms linking placental insufficiency with impaired islet development and β-cell function.
Keywords: IUGR; islet; pancreas; β-cell.
© 2017 Society for Endocrinology.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Abuzgaia AM, Hardy DB, Arany E. Regulation of postnatal pancreatic Pdx1 and downstream target genes after gestational exposure to protein restriction in rats. Reproduction. 2015;149:293–303. - PubMed
-
- Aldoretta PW, Hay WW. Effect of glucose supply on ovine uteroplacental glucose metabolism. Am J Physiol Regul Integr Comp Physiol. 1999;277:R947–R958. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

