Inhibitor-induced oxidation of the nucleus and cytosol in Arabidopsis thaliana: implications for organelle to nucleus retrograde signalling
- PMID: 28808105
- PMCID: PMC5566886
- DOI: 10.1098/rstb.2016.0392
Inhibitor-induced oxidation of the nucleus and cytosol in Arabidopsis thaliana: implications for organelle to nucleus retrograde signalling
Abstract
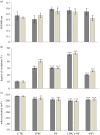
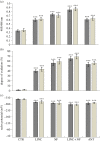
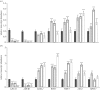
Concepts of organelle-to-nucleus signalling pathways are largely based on genetic screens involving inhibitors of chloroplast and mitochondrial functions such as norflurazon, lincomycin (LINC), antimycin A (ANT) and salicylhydroxamic acid. These inhibitors favour enhanced cellular oxidation, but their precise effects on the cellular redox state are unknown. Using the in vivo reduction-oxidation (redox) reporter, roGFP2, inhibitor-induced changes in the glutathione redox potentials of the nuclei and cytosol were measured in Arabidopsis thaliana root, epidermal and stomatal guard cells, together with the expression of nuclear-encoded chloroplast and mitochondrial marker genes. All the chloroplast and mitochondrial inhibitors increased the degree of oxidation in the nuclei and cytosol. However, inhibitor-induced oxidation was less marked in stomatal guard cells than in epidermal or root cells. Moreover, LINC and ANT caused a greater oxidation of guard cell nuclei than the cytosol. Chloroplast and mitochondrial inhibitors significantly decreased the abundance of LHCA1 and LHCB1 transcripts. The levels of WHY1, WHY3 and LEA5 transcripts were increased in the presence of inhibitors. Chloroplast inhibitors decreased AOXA1 mRNA levels, while mitochondrial inhibitors had the opposite effect. Inhibitors that are used to characterize retrograde signalling pathways therefore have similar general effects on cellular redox state and gene expression.This article is part of the themed issue 'Enhancing photosynthesis in crop plants: targets for improvement'.
Keywords: alternative oxidase; antimycin A; lincomycin; norflurazon; nuclear-encoded mitochondrial proteins; photosynthesis-associated nuclear genes.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures


Similar articles
-
Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge.Biochim Biophys Acta. 2009 May;1787(5):468-75. doi: 10.1016/j.bbabio.2009.01.020. Epub 2009 Feb 3. Biochim Biophys Acta. 2009. PMID: 19366606
-
Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana.Plant Cell Environ. 2015 Feb;38(2):266-79. doi: 10.1111/pce.12252. Epub 2014 Jan 13. Plant Cell Environ. 2015. PMID: 24329757
-
Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer.Plant J. 2007 Dec;52(5):973-86. doi: 10.1111/j.1365-313X.2007.03280.x. Epub 2007 Sep 22. Plant J. 2007. PMID: 17892447
-
Chloroplast-associated molecular patterns as concept for fine-tuned operational retrograde signalling.Philos Trans R Soc Lond B Biol Sci. 2020 Jun 22;375(1801):20190443. doi: 10.1098/rstb.2019.0443. Epub 2020 May 4. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32362264 Free PMC article. Review.
-
An Insight Into the Mechanism of Plant Organelle Genome Maintenance and Implications of Organelle Genome in Crop Improvement: An Update.Front Cell Dev Biol. 2021 Aug 10;9:671698. doi: 10.3389/fcell.2021.671698. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34447743 Free PMC article. Review.
Cited by
-
Redox regulation, thioredoxins, and glutaredoxins in retrograde signalling and gene transcription.J Exp Bot. 2023 Oct 13;74(19):5955-5969. doi: 10.1093/jxb/erad270. J Exp Bot. 2023. PMID: 37453076 Free PMC article. Review.
-
Circadian and diel regulation of photosynthesis in the bryophyte Marchantia polymorpha.Plant Cell Environ. 2022 Aug;45(8):2381-2394. doi: 10.1111/pce.14364. Epub 2022 Jun 3. Plant Cell Environ. 2022. PMID: 35611455 Free PMC article.
-
Reactive oxygen species, oxidative signaling and the regulation of photosynthesis.Environ Exp Bot. 2018 Oct;154:134-142. doi: 10.1016/j.envexpbot.2018.05.003. Environ Exp Bot. 2018. PMID: 30283160 Free PMC article. Review.
-
Photosynthesis solutions to enhance productivity.Philos Trans R Soc Lond B Biol Sci. 2017 Sep 26;372(1730):20160374. doi: 10.1098/rstb.2016.0374. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28808094 Free PMC article.
-
Ascorbate-mediated regulation of growth, photoprotection, and photoinhibition in Arabidopsis thaliana.J Exp Bot. 2018 May 19;69(11):2823-2835. doi: 10.1093/jxb/ery170. J Exp Bot. 2018. PMID: 29726917 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

