Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity
- PMID: 28808109
- PMCID: PMC5566890
- DOI: 10.1098/rstb.2016.0543
Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity
Abstract
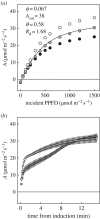
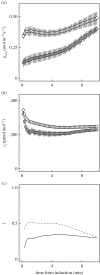
Wheat is the second most important direct source of food calories in the world. After considerable improvement during the Green Revolution, increase in genetic yield potential appears to have stalled. Improvement of photosynthetic efficiency now appears a major opportunity in addressing the sustainable yield increases needed to meet future food demand. Effort, however, has focused on increasing efficiency under steady-state conditions. In the field, the light environment at the level of individual leaves is constantly changing. The speed of adjustment of photosynthetic efficiency can have a profound effect on crop carbon gain and yield. Flag leaves of wheat are the major photosynthetic organs supplying the grain of wheat, and will be intermittently shaded throughout a typical day. Here, the speed of adjustment to a shade to sun transition in these leaves was analysed. On transfer to sun conditions, the leaf required about 15 min to regain maximum photosynthetic efficiency. In vivo analysis based on the responses of leaf CO2 assimilation (A) to intercellular CO2 concentration (ci) implied that the major limitation throughout this induction was activation of the primary carboxylase of C3 photosynthesis, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). This was followed in importance by stomata, which accounted for about 20% of the limitation. Except during the first few seconds, photosynthetic electron transport and regeneration of the CO2 acceptor molecule, ribulose-1,5-bisphosphate (RubP), did not affect the speed of induction. The measured kinetics of Rubisco activation in the sun and de-activation in the shade were predicted from the measurements. These were combined with a canopy ray tracing model that predicted intermittent shading of flag leaves over the course of a June day. This indicated that the slow adjustment in shade to sun transitions could cost 21% of potential assimilation.This article is part of the themed issue 'Enhancing photosynthesis in crop plants: targets for improvement'.
Keywords: Rubisco; Rubisco activase; crop yield improvement; food security; photosynthetic induction; wheat.
© 2017 The Authors.
Conflict of interest statement
We have no competing interests.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

