Listeria monocytogenes Has Both Cytochrome bd-Type and Cytochrome aa 3-Type Terminal Oxidases, Which Allow Growth at Different Oxygen Levels, and Both Are Important in Infection
- PMID: 28808161
- PMCID: PMC5649020
- DOI: 10.1128/IAI.00354-17
Listeria monocytogenes Has Both Cytochrome bd-Type and Cytochrome aa 3-Type Terminal Oxidases, Which Allow Growth at Different Oxygen Levels, and Both Are Important in Infection
Abstract
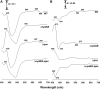
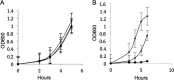
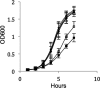
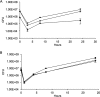
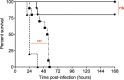
Listeria monocytogenes is a foodborne pathogen responsible for a number of life-threatening infections of humans. During an infection, it invades epithelial cells before spreading from the intestine to the cells of the liver and spleen. This requires an ability to adapt to varying oxygen levels. Here, we demonstrate that L. monocytogenes has two terminal oxidases, a cytochrome bd-type (CydAB) and a cytochrome aa 3-type menaquinol (QoxAB) oxidase, and that both are used for respiration under different oxygen tensions. Furthermore, we show that possession of both terminal oxidases is important in infection. In air, the CydAB bd-type oxidase is essential for aerobic respiration and intracellular replication, and cydAB mutants are highly attenuated in mice. In contrast, the QoxAB aa 3-type oxidase is required neither for aerobic respiration in air nor for intracellular growth. However, the qoxAB mutants are attenuated in mice, with a delay in the onset of disease signs and with increased survival time, indicating a role for the QoxAB aa 3-type oxidase in the initial stages of infection. Growth of bacteria under defined oxygen conditions revealed that at 1% (vol/vol), both oxidases are functional, and the presence of either is sufficient for aerobic respiration and intracellular replication. However, at 0.2% (vol/vol), both oxidases are necessary for maximum growth. These findings are consistent with the ability of L. monocytogenes to switch between terminal oxidases under different oxygen conditions, providing exquisite adaptation to different conditions encountered within the infected host.
Keywords: Listeria monocytogenes; cytochromes; foodborne pathogens; host-pathogen interactions; oxygen.
Copyright © 2017 Corbett et al.
Figures

References
-
- Lyautey E, Lapen DR, Wilkes G, McCleary K, Pagotto F, Tyler K, Hartmann A, Piveteau P, Rieu A, Robertson WJ, Medeiros DT, Edge TA, Gannon V, Topp E. 2007. Distribution and characteristics of Listeria monocytogenes isolates from surface waters of the South Nation River watershed, Ontario, Canada. Appl Environ Microbiol 73:5401–5410. doi: 10.1128/AEM.00354-07. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

