Safety, immunogenicity, and cross-species protection of a plasmid DNA encoding Plasmodium falciparum SERA5 polypeptide, microbial epitopes and chemokine genes in mice and olive baboons
- PMID: 28808204
- PMCID: PMC5548993
- DOI: 10.7555/JBR.31.20160025
Safety, immunogenicity, and cross-species protection of a plasmid DNA encoding Plasmodium falciparum SERA5 polypeptide, microbial epitopes and chemokine genes in mice and olive baboons
Abstract
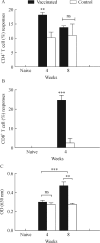
Incorporation of biomolecular epitopes to malarial antigens should be explored in the development of strain-transcending malarial vaccines. The present study sought to determine safety, immunogenicity and cross-species efficacy ofPlasmodium falciparum serine repeat antigen 5 polypeptide co-expressed with epitopes of Bacille-Calmette Guerin (BCG), tetanus toxoid (TT) and a chemokine gene. Olive baboons and BALB/c mice were randomly assigned into vaccine and control groups. The vaccine group animals were primed and boosted twice with pIRES plasmids encoding the SERA5+ BCG+ TT alone, or with either CCL5 or CCL20 and the control group with pIRES plasmid vector backbone. Mice and baboons were challenged withP. berghei ANKA and P. knowlesi H strain parasites, respectively. Safety was determined by observing for injection sites reactogenicities, hematology and clinical chemistry. Parasitaemia and survivorship profiles were used to determine cross-species efficacy, and T cell phenotypes, Th1-, Th2-type, T-regulatory immune responses and antibody responses were assessed to determine vaccine immunogenicity. The pSeBCGTT plasmid DNA vaccines were safe and induced Th1-, Th2-type, and T-regulatory responses vaccinated animals showed enhanced CD4+ (P<0.01), CD 8+ T cells (P<0.001) activation and IgG anti-SE36 antibodies responses (P<0.001) at week 4 and 8 post vaccination compared to the control group. Vaccinated mice had a 31.45-68.69% cumulative parasite load reduction and 60% suppression in baboons (P<0.05) and enhanced survivorship (P<0.001) with no clinical signs of malaria compared to the control group. The results showed that the vaccines were safe, immunogenic and conferred partial cross-species protection.
Figures



Similar articles
-
African-specific polymorphisms in Plasmodium falciparum serine repeat antigen 5 in Uganda and Burkina Faso clinical samples do not interfere with antibody response to BK-SE36 vaccination.Front Cell Infect Microbiol. 2022 Dec 16;12:1058081. doi: 10.3389/fcimb.2022.1058081. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36590593 Free PMC article.
-
Increased B and T Cell Responses in M. bovis Bacille Calmette-Guérin Vaccinated Pigs Co-Immunized with Plasmid DNA Encoding a Prototype Tuberculosis Antigen.PLoS One. 2015 Jul 14;10(7):e0132288. doi: 10.1371/journal.pone.0132288. eCollection 2015. PLoS One. 2015. PMID: 26172261 Free PMC article.
-
Evidences of protection against blood-stage infection of Plasmodium falciparum by the novel protein vaccine SE36.Parasitol Int. 2010 Sep;59(3):380-6. doi: 10.1016/j.parint.2010.05.002. Epub 2010 May 20. Parasitol Int. 2010. PMID: 20493274 Clinical Trial.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes.J Biotechnol. 1996 Jan 26;44(1-3):91-6. doi: 10.1016/0168-1656(95)00118-2. J Biotechnol. 1996. PMID: 8717391 Review.
References
-
- WHO. World Malaria Report[EB/OL]. Geneva; 2014, http://www.who.int/malaria/publications/world_malaria_report_2014/en/.
-
- Birkett AJ, Moorthy VS, Loucq C, et al. Malaria vaccine R&D in the decade of vaccines: breakthroughs, challenges and opportunities[J]. Vaccine, 2013, 31(Suppl 2): B233–B243 . - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

