Effects of One-Week Empirical Antibiotic Therapy on the Early Development of Gut Microbiota and Metabolites in Preterm Infants
- PMID: 28808302
- PMCID: PMC5556106
- DOI: 10.1038/s41598-017-08530-9
Effects of One-Week Empirical Antibiotic Therapy on the Early Development of Gut Microbiota and Metabolites in Preterm Infants
Abstract
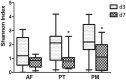
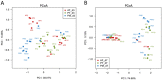
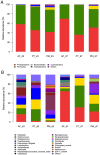
The early postnatal period is the most dynamic and vulnerable stage in the assembly of intestinal microbiota. Antibiotics are commonly prescribed to newborn preterm babies and are frequently used for a prolonged duration in China. We hypothesized that the prolonged antibiotic therapy would affect the early development of intestinal microbiota and their metabolites. To test this hypothesis, we analyzed the stool microbiota and metabolites in 36 preterm babies with or without antibiotic treatment. These babies were divided into three groups, including two groups treated with the combination of penicillin and moxalactam or piperacillin-tazobactam for 7 days, and the other group was free of antibiotics. Compared to the antibiotic-free group, both antibiotic-treated groups had distinct gut microbial communities and metabolites, including a reduction of bacterial diversity and an enrichment of harmful bacteria such as Streptococcus and Pseudomonas. In addition, there was a significant difference in the composition of gut microbiota and their metabolites between the two antibiotic-treated groups, where the piperacillin-tazobactam treatment group showed an overgrowth of Enterococcus. These findings suggest that prolonged antibiotic therapy affects the early development of gut microbiota in preterm infants, which should be considered when prescribing antibiotics for this population.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Prenatal and postnatal antibiotic exposure influences the gut microbiota of preterm infants in neonatal intensive care units.Ann Clin Microbiol Antimicrob. 2018 Mar 19;17(1):9. doi: 10.1186/s12941-018-0264-y. Ann Clin Microbiol Antimicrob. 2018. PMID: 29554907 Free PMC article.
-
Effects of β-Lactam Antibiotics on Gut Microbiota Colonization and Metabolites in Late Preterm Infants.Curr Microbiol. 2020 Dec;77(12):3888-3896. doi: 10.1007/s00284-020-02198-7. Epub 2020 Sep 24. Curr Microbiol. 2020. PMID: 32970172
-
Association between duration of intravenous antibiotic administration and early-life microbiota development in late-preterm infants.Eur J Clin Microbiol Infect Dis. 2018 Mar;37(3):475-483. doi: 10.1007/s10096-018-3193-y. Epub 2018 Jan 24. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29368074 Free PMC article.
-
Antibiotic perturbation of the preterm infant gut microbiome and resistome.Gut Microbes. 2016 Sep 2;7(5):443-9. doi: 10.1080/19490976.2016.1218584. Epub 2016 Jul 29. Gut Microbes. 2016. PMID: 27472377 Free PMC article. Review.
-
Antibiotics and the developing infant gut microbiota and resistome.Curr Opin Microbiol. 2015 Oct;27:51-6. doi: 10.1016/j.mib.2015.07.007. Epub 2015 Aug 1. Curr Opin Microbiol. 2015. PMID: 26241507 Free PMC article. Review.
Cited by
-
Probiotics and gut microbiota modulation: implications for skin health and disease management.Arch Microbiol. 2025 Feb 23;207(3):68. doi: 10.1007/s00203-025-04267-6. Arch Microbiol. 2025. PMID: 39988585 Review.
-
Early antibiotic exposure in very-low birth weight infants and infection risk at 3-7 days after birth.J Perinatol. 2023 Sep;43(9):1158-1165. doi: 10.1038/s41372-023-01737-x. Epub 2023 Jul 25. J Perinatol. 2023. PMID: 37491474 Free PMC article.
-
Differences in Compositions of Gut Bacterial Populations and Bacteriophages in 5-11 Year-Olds Born Preterm Compared to Full Term.Front Cell Infect Microbiol. 2020 Jun 16;10:276. doi: 10.3389/fcimb.2020.00276. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32612960 Free PMC article.
-
Acquisition, Divergence, and Personalization of the Female Perineal Microbiomes Are Driven by Developmental Milestones and Disrupted by Urinary Tract Infection: A Pilot Study.Front Pediatr. 2020 Dec 8;8:542413. doi: 10.3389/fped.2020.542413. eCollection 2020. Front Pediatr. 2020. PMID: 33364220 Free PMC article.
-
Impact of Early-Life Microbiota on Immune System Development and Allergic Disorders.Biomedicines. 2025 Jan 7;13(1):121. doi: 10.3390/biomedicines13010121. Biomedicines. 2025. PMID: 39857705 Free PMC article. Review.
References
-
- Sharma R, Young C, Mshvildadze M, Neu J. Intestinal Microbiota: Does It Play a Role in Diseases of the Neonate? NeoReviews. 2009;10:e166–e179. doi: 10.1542/neo.10-4-e166. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

