Inhibition of mTOR signaling Confers Protection against Cerebral Ischemic Injury in Acute Hyperglycemic Rats
- PMID: 28808420
- PMCID: PMC5555105
- DOI: 10.7150/ijbs.18976
Inhibition of mTOR signaling Confers Protection against Cerebral Ischemic Injury in Acute Hyperglycemic Rats
Abstract
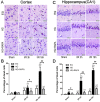
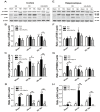
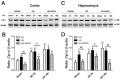
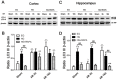
Hyperglycemia is known to exacerbate neuronal death resulted from cerebral ischemia. The mechanisms are not fully understood. The mammalian target of rapamycin (mTOR) pathway regulates cell growth, division and apoptosis. Recent studies suggest that activation of mTOR may mediate ischemic brain damage. The objective of the present experiment is to explore whether mTOR mediates ischemic brain damage in acute hyperglycemic animals. Rats were subjected to 10 min of forebrain ischemia under euglycemic, hyperglycemic and rapamycin-treated hyperglycemic conditions. The rat brain samples were collected from the cortex and hippocampi after 3h and 16h of reperfusion. The results showed that hyperglycemia significantly increased neuronal death in the cortex and hippocampus and the exacerbation effect of hyperglycemia was associated with further activation of mTOR under control and/or ischemic conditions. Inhibition of mTOR with rapamycin ameliorated the damage and suppressed hyperglycemia-elevated p-MTOR, p-P70S6K and p-S6. In addition, hyperglycemia per se increased the levels of cytosolic cytochrome c and autophagy marker LC3-II, while rapamycin alleviated these alterations. It is concluded that activation of mTOR signaling may play a detrimental role in mediating the aggravating effect of hyperglycemia on cerebral ischemia.
Keywords: Cerebral ischemia; Hyperglycemia; Pathology; Rapamycin; Rat.; mTOR.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Prevention of post-ischemic seizure by rapamycin is associated with deactivation of mTOR and ERK1/2 pathways in hyperglycemic rats.Biochem Biophys Res Commun. 2019 Nov 26;520(1):47-53. doi: 10.1016/j.bbrc.2019.09.096. Epub 2019 Sep 26. Biochem Biophys Res Commun. 2019. PMID: 31564412
-
Inhibition of mTOR Pathway by Rapamycin Reduces Brain Damage in Rats Subjected to Transient Forebrain Ischemia.Int J Biol Sci. 2015 Nov 24;11(12):1424-35. doi: 10.7150/ijbs.12930. eCollection 2015. Int J Biol Sci. 2015. PMID: 26681922 Free PMC article.
-
Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling.Int J Biol Sci. 2016 Jul 18;12(8):1032-40. doi: 10.7150/ijbs.15624. eCollection 2016. Int J Biol Sci. 2016. PMID: 27489506 Free PMC article.
-
The rationale of targeting mammalian target of rapamycin for ischemic stroke.Cell Signal. 2013 Jul;25(7):1598-607. doi: 10.1016/j.cellsig.2013.03.017. Epub 2013 Apr 3. Cell Signal. 2013. PMID: 23563259 Review.
-
The role of mTOR signaling pathway in spinal cord injury.Cell Cycle. 2012 Sep 1;11(17):3175-9. doi: 10.4161/cc.21262. Epub 2012 Aug 16. Cell Cycle. 2012. PMID: 22895182 Free PMC article. Review.
Cited by
-
A 2-Min Transient Ischemia Confers Cerebral Ischemic Tolerance in Non-Obese Gerbils, but Results in Neuronal Death in Obese Gerbils by Increasing Abnormal mTOR Activation-Mediated Oxidative Stress and Neuroinflammation.Cells. 2019 Sep 22;8(10):1126. doi: 10.3390/cells8101126. Cells. 2019. PMID: 31546722 Free PMC article.
-
Experimental and clinical tests of FDA-approved kinase inhibitors for the treatment of neurological disorders (update 2024).Explor Drug Sci. 2025;3:1008116. doi: 10.37349/eds.2025.1008116. Epub 2025 Jul 1. Explor Drug Sci. 2025. PMID: 40708570 Free PMC article.
-
Dexmedetomidine attenuates neuronal injury induced by cerebral ischemia‑reperfusion by regulating miR‑199a.Mol Med Rep. 2021 Aug;24(2):574. doi: 10.3892/mmr.2021.12213. Epub 2021 Jun 10. Mol Med Rep. 2021. PMID: 34109426 Free PMC article.
-
MicroRNA-216b targets HK2 to potentiate autophagy and apoptosis of breast cancer cells via the mTOR signaling pathway.Int J Biol Sci. 2021 Jul 13;17(11):2970-2983. doi: 10.7150/ijbs.48933. eCollection 2021. Int J Biol Sci. 2021. PMID: 34345220 Free PMC article.
-
Deletion of Mitochondrial Uncoupling Protein 2 Exacerbates Mitochondrial Damage in Mice Subjected to Cerebral Ischemia and Reperfusion Injury under both Normo- and Hyperglycemic Conditions.Int J Biol Sci. 2020 Aug 25;16(15):2788-2802. doi: 10.7150/ijbs.48204. eCollection 2020. Int J Biol Sci. 2020. PMID: 33061796 Free PMC article.
References
-
- Li PA, Uchino H, Elmer E, Siesjo BK. Amelioration by cyclosporin A of brain damage following 5 or 10 min of ischemia in rats subjected to preischemic hyperglycemia. Brain Res. 1997;753:133–40. - PubMed
-
- Siesjö BK, Katsura KI, Kristian T, Li PA, Siesjö P. Molecular mechanisms of acidosis-mediated damage. Acta Neurochir Suppl. 1996;66:8–14. - PubMed
-
- Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11:261–71. - PubMed
-
- Luitse MJ, Velthuis BK, Kappelle LJ, van der Graaf Y, Biessels GJ. Chronic hyperglycemia is related to poor functional outcome after acute ischemic stroke. Int J Stroke; 2016. DOI: 10.1177/1747493016676619. [Epub ahead of print] - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

