A highly selective near-infrared fluorescent probe for imaging H2Se in living cells and in vivo
- PMID: 28808528
- PMCID: PMC5531029
- DOI: 10.1039/c5sc03471j
A highly selective near-infrared fluorescent probe for imaging H2Se in living cells and in vivo
Abstract
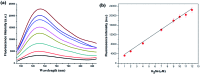
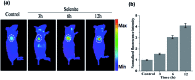
Hydrogen selenide (H2Se), a highly reactive Se species, is an important selenium metabolism intermediate involved in many physiological and pathological processes. This compound is of scientific interest with regard to the real-time monitoring of H2Se in living cells and in vivo to understand the anti-cancer mechanism of selenium. However, monitoring H2Se in living cells is still challenging due to the lack of straight forward, highly selective and rapid methods. Here, we developed a novel small-molecule fluorescent probe, NIR-H2Se, for imaging endogenous H2Se. NIR-H2Se exhibited high selectivity toward H2Se over selenocysteine (Sec), H2S and small molecule thiols and was successfully used to image the H2Se content in HepG2 cells during Na2SeO3-induced apoptosis. Increased H2Se content and reduced ROS levels were observed under hypoxic conditions compared to normoxic conditions, which indicated that the cell apoptosis induced by Na2SeO3 under a hypoxic environment is via a non-oxidative stress mechanism. Thus, this probe should serve as a powerful tool for exploring the physiological function of H2Se and Se anticancer mechanisms in a variety of physiological and pathological contexts.
Figures





Similar articles
-
New Insights into Oxidative and Reductive Stress Responses and Their Relation to the Anticancer Activity of Selenium-Containing Compounds as Hydrogen Selenide Donors.Biology (Basel). 2023 Jun 17;12(6):875. doi: 10.3390/biology12060875. Biology (Basel). 2023. PMID: 37372159 Free PMC article. Review.
-
Highly Selective Fluorescent Probe for Imaging H2Se in Living Cells and in Vivo Based on the Disulfide Bond.Anal Chem. 2017 Jan 3;89(1):688-693. doi: 10.1021/acs.analchem.6b03136. Epub 2016 Dec 15. Anal Chem. 2017. PMID: 27976866
-
Double-ratiometric fluorescence imaging of H2Se and O2˙- under hypoxia for exploring Na2SeO3-induced HepG2 cells' apoptosis.RSC Adv. 2018 Dec 7;8(71):40984-40988. doi: 10.1039/c8ra08142e. eCollection 2018 Dec 4. RSC Adv. 2018. PMID: 35557927 Free PMC article.
-
H2Se Induces Reductive Stress in HepG2 Cells and Activates Cell Autophagy by Regulating the Redox of HMGB1 Protein under Hypoxia.Theranostics. 2019 Feb 28;9(6):1794-1808. doi: 10.7150/thno.31841. eCollection 2019. Theranostics. 2019. PMID: 31037139 Free PMC article.
-
A review of bioselenol-specific fluorescent probes: Synthesis, properties, and imaging applications.Anal Chim Acta. 2020 May 8;1110:141-150. doi: 10.1016/j.aca.2020.03.027. Epub 2020 Mar 17. Anal Chim Acta. 2020. PMID: 32278389 Review.
Cited by
-
A specific fluorescent probe reveals compromised activity of methionine sulfoxide reductases in Parkinson's disease.Chem Sci. 2017 Apr 1;8(4):2966-2972. doi: 10.1039/c6sc04708d. Epub 2017 Jan 27. Chem Sci. 2017. PMID: 28451363 Free PMC article.
-
New Insights into Oxidative and Reductive Stress Responses and Their Relation to the Anticancer Activity of Selenium-Containing Compounds as Hydrogen Selenide Donors.Biology (Basel). 2023 Jun 17;12(6):875. doi: 10.3390/biology12060875. Biology (Basel). 2023. PMID: 37372159 Free PMC article. Review.
-
An Examination of Chemical Tools for Hydrogen Selenide Donation and Detection.Molecules. 2024 Aug 15;29(16):3863. doi: 10.3390/molecules29163863. Molecules. 2024. PMID: 39202942 Free PMC article. Review.
-
Excess selenium intake is associated with microalbuminuria in female but not in male among adults with obesity: Results from NHANES 2009-2018.Front Nutr. 2023 Jan 25;10:1043395. doi: 10.3389/fnut.2023.1043395. eCollection 2023. Front Nutr. 2023. PMID: 36761214 Free PMC article.
-
Seleno-Metabolites and Their Precursors: A New Dawn for Several Illnesses?Metabolites. 2022 Sep 16;12(9):874. doi: 10.3390/metabo12090874. Metabolites. 2022. PMID: 36144278 Free PMC article. Review.
References
-
- Reeves M. A., Hoffmann P. R. Cell. Mol. Life Sci. 2009;66:2457–2478. - PMC - PubMed
- Misra S., Peak D., Chen N., Hamilton C., Niyogi S. Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol. 2012;155:560–565. - PubMed
- Fairweather-Tait S. J., Bao Y., Broadley M. R., Collings R., Ford D., Hesketh J. E., Hurst R. Antioxid. Redox Signaling. 2011;14:1337–1383. - PubMed
-
- Weekley C. M., Aitken J. B., Vogt S., Finney L. A., Paterson D. J., de Jonge M. D., Howard D. L., Musgrave I. F., Harris H. H. Biochemistry. 2011;50:1641–1650. - PubMed
- Duffield-Lillico A. J., Reid M. E., Turnbull B. W., Combs Jr G. F., Slate E. H., Fischbach L. A., Marshall J. R., Clark L. C. Cancer Epidemiol., Biomarkers Prev. 2002;11:630–639. - PubMed
- Weekley C. M., Aitken J. B., Vogt S., Finney L. A., Paterson D. J., de Jonge M. D., Howard D. L., Witting P. K., Musgrave I. F., Harris H. H. J. Am. Chem. Soc. 2011;133:18272–18279. - PMC - PubMed
-
- Weekley C. M., Harris H. H. Chem. Soc. Rev. 2013;42:8870–8894. - PubMed
- Clark L. C., Combs G. F., Turnbull B. W., Slate E. H., Chalker D. K., Chow J., Davis L. S., Glover R. A., Graham G. F., Gross E. G. J. Am. Med. Assoc. 1996;276:1957–1963. - PubMed
- Lipp-man S. M., Klein E. A., Goodman P. J., Lucia M. S., Thompson I. M., Ford L. G., Parnes H. L., Minasian L. M., Gaziano J. M., Hartline J. A. J. Am. Med. Assoc. 2009;301:39–51. - PubMed
-
- Combs G., Gray W. Pharmacol. Ther. 1998;79:179–192. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

