Covalent modification of biological targets with natural products through Paal-Knorr pyrrole formation
- PMID: 28808718
- PMCID: PMC5759776
- DOI: 10.1039/c7np00024c
Covalent modification of biological targets with natural products through Paal-Knorr pyrrole formation
Abstract
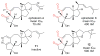
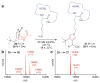
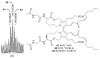
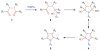
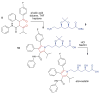
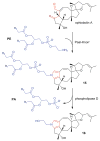
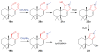
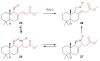
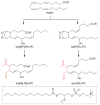
Covering: up to June 2017Natural products and endogenous metabolites engage specific targets within tissues and cells through complex mechanisms. This review examines the extent to which natural systems have adopted the Paal-Knorr reaction to engage nucleophilic amine groups within biological targets. Current understanding of this mode of reactivity is limited by only a few examples of this reaction in a biological context. This highlight is intended to stimulate the scientific community to identify potential research directions and applications of the Paal-Knorr reaction in native and engineered biological systems.
Figures
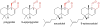




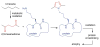

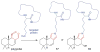




Similar articles
-
A novel one-pot pyrrole synthesis via a coupling-isomerization-Stetter-Paal-Knorr sequence.Org Lett. 2001 Oct 18;3(21):3297-300. doi: 10.1021/ol0165185. Org Lett. 2001. PMID: 11594818
-
Flow synthesis using gaseous ammonia in a Teflon AF-2400 tube-in-tube reactor: Paal-Knorr pyrrole formation and gas concentration measurement by inline flow titration.Org Biomol Chem. 2012 Aug 14;10(30):5774-9. doi: 10.1039/c2ob25407g. Epub 2012 Apr 24. Org Biomol Chem. 2012. PMID: 22532036
-
Solution-phase synthesis of a tricyclic pyrrole-2-carboxamide discovery library applying a stetter-Paal-Knorr reaction sequence.J Comb Chem. 2006 May-Jun;8(3):368-80. doi: 10.1021/cc050160c. J Comb Chem. 2006. PMID: 16677007 Free PMC article.
-
Green Synthesis of Bioactive Pyrrole Derivatives via Heterogeneous Catalysts Since 2010.Curr Top Med Chem. 2025;25(5):461-492. doi: 10.2174/0115680266307696240708115422. Curr Top Med Chem. 2025. PMID: 39069813 Review.
-
Synthesis of natural products containing the pyrrolic ring.Nat Prod Rep. 2010 Jan;27(12):1801-39. doi: 10.1039/c0np00014k. Epub 2010 Oct 8. Nat Prod Rep. 2010. PMID: 20936222 Review. No abstract available.
Cited by
-
A Paal-Knorr agent for chemoproteomic profiling of targets of isoketals in cells.Chem Sci. 2021 Oct 15;12(43):14557-14563. doi: 10.1039/d1sc02230j. eCollection 2021 Nov 10. Chem Sci. 2021. PMID: 34881007 Free PMC article.
-
Lessons in Organic Fluorescent Probe Discovery.Chembiochem. 2021 Nov 16;22(22):3109-3139. doi: 10.1002/cbic.202100171. Epub 2021 Jun 23. Chembiochem. 2021. PMID: 34062039 Free PMC article. Review.
-
Ribose conversion with amino acids into pyrraline platform chemicals - expeditious synthesis of diverse pyrrole-fused alkaloid compounds.RSC Adv. 2021 Sep 23;11(50):31511-31525. doi: 10.1039/d1ra06110k. eCollection 2021 Sep 21. RSC Adv. 2021. PMID: 35496880 Free PMC article.
-
Observation and Kinetic Characterization of Transient Schiff Base Intermediates by CEST NMR Spectroscopy.Angew Chem Int Ed Engl. 2019 Oct 21;58(43):15309-15312. doi: 10.1002/anie.201908416. Epub 2019 Sep 17. Angew Chem Int Ed Engl. 2019. PMID: 31449352 Free PMC article.
-
CYP27A1-dependent anti-melanoma activity of limonoid natural products targets mitochondrial metabolism.Cell Chem Biol. 2021 Oct 21;28(10):1407-1419.e6. doi: 10.1016/j.chembiol.2021.03.004. Epub 2021 Mar 31. Cell Chem Biol. 2021. PMID: 33794192 Free PMC article.
References
-
- Böttcher T, Pitscheider M, Sieber SA. Angew Chem, Int Ed. 2010;49:2680. - PubMed
- Drahl C, Cravatt BF, Sorensen EJ. Angew Chem, Int Ed. 2005;44:5788. - PubMed
- Jöst C, Nitsche C, Scholz T, Roux L, Klein CD. J Med Chem. 2014;57:7590. - PubMed
- Njuguna NM, Masimirembwa C, Chibale K. J Nat Prod. 2012;75:507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

