Germ cell connectivity enhances cell death in response to DNA damage in the Drosophila testis
- PMID: 28809158
- PMCID: PMC5577909
- DOI: 10.7554/eLife.27960
Germ cell connectivity enhances cell death in response to DNA damage in the Drosophila testis
Abstract
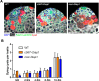
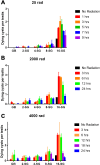
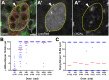
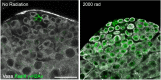
Two broadly known characteristics of germ cells in many organisms are their development as a 'cyst' of interconnected cells and their high sensitivity to DNA damage. Here we provide evidence that in the Drosophila testis, connectivity serves as a mechanism that confers to spermatogonia a high sensitivity to DNA damage. We show that all spermatogonia within a cyst die synchronously even when only a subset of them exhibit detectable DNA damage. Mutants of the fusome, an organelle that is known to facilitate intracyst communication, compromise synchronous spermatogonial death and reduces overall germ cell death. Our data indicate that a death-promoting signal is shared within the cyst, leading to death of the entire cyst. Taken together, we propose that intercellular connectivity supported by the fusome uniquely increases the sensitivity of the germline to DNA damage, thereby protecting the integrity of gamete genomes that are passed on to the next generation.
Keywords: D. melanogaster; DNA damage; developmental biology; fusome; germ cell cyst; germ cells; stem cells.
Conflict of interest statement
Reviewing editor,
No competing interests declared.
Figures







References
-
- Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, Rio DC, Rubin GM. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Molecular and Cellular Biology. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

