A Review on the Synthesis and Applications of Mesostructured Transition Metal Phosphates
- PMID: 28809304
- PMCID: PMC5452118
- DOI: 10.3390/ma6010217
A Review on the Synthesis and Applications of Mesostructured Transition Metal Phosphates
Abstract
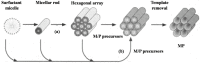
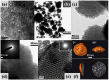
Considerable efforts have been devoted to extending the range of the elemental composition of mesoporous materials since the pioneering work of the M41S family of ordered mesoporous silica by Mobil researchers. The synthesis of transition metal-containing mesostructured materials with large surface area and high porosity has drawn great attention for its potential applications in acid and redox catalysis, photocatalysis, proton conducting devices, environmental restoration and so on. Thus, various transition metals-containing mesoporous materials, including transition metal-substituted mesoporous silicates, mesostructured transition metal oxides and transition metal phosphates (TMP), have been documented in the literature. Among these, mesostructured TMP materials are less studied, but possess some unique features, partly because of the easy and facile functionalization of PO₄ and/or P-OH groups, rendering them interesting functional materials. This review first introduced the general synthesis strategies for manufacturing mesostructured TMP materials, as well as advantages and disadvantages of the respective method; then, we surveyed the ongoing developments of fabrication and application of the TMP materials in three groups on the basis of their components and application fields. Future perspectives on existing problems related to the present synthesis routes and further modifying of the functional groups for the purpose of tailoring special physical-chemical properties to meet wide application requirements were also provided in the last part.
Keywords: application; mesostructure; synthesis method; transition metal phosphate.
Figures





Similar articles
-
Recent advances in synthesis and applications of transition metal containing mesoporous molecular sieves.Angew Chem Int Ed Engl. 2002 Jan 18;41(2):215-29. doi: 10.1002/1521-3773(20020118)41:2<214::aid-anie214>3.0.co;2-d. Angew Chem Int Ed Engl. 2002. PMID: 12491401
-
Fundamentals and recent progress relating to the fabrication, functionalization and characterization of mesostructured materials using diverse synthetic methodologies.RSC Adv. 2020 Apr 24;10(28):16431-16456. doi: 10.1039/d0ra00440e. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35517916 Free PMC article. Review.
-
Electroinduced Surfactant Self-Assembly Driven to Vertical Growth of Oriented Mesoporous Films.Acc Chem Res. 2021 Sep 21;54(18):3563-3575. doi: 10.1021/acs.accounts.1c00233. Epub 2021 Sep 1. Acc Chem Res. 2021. PMID: 34469107
-
A general synthetic approach for ordered mesoporous metal sulfides.J Am Chem Soc. 2014 Jun 25;136(25):8895-8. doi: 10.1021/ja504407e. Epub 2014 Jun 10. J Am Chem Soc. 2014. PMID: 24904959
-
Mesoporous materials and electrochemistry.Chem Soc Rev. 2013 May 7;42(9):4098-140. doi: 10.1039/c2cs35322a. Epub 2013 Jan 18. Chem Soc Rev. 2013. PMID: 23334166 Review.
Cited by
-
About the Dominance of Mesopores in Physisorption in Amorphous Materials.Molecules. 2021 Nov 27;26(23):7190. doi: 10.3390/molecules26237190. Molecules. 2021. PMID: 34885773 Free PMC article.
-
Crystal Structures of Two Titanium Phosphate-Based Proton Conductors: Ab Initio Structure Solution and Materials Properties.Inorg Chem. 2022 Feb 7;61(5):2379-2390. doi: 10.1021/acs.inorgchem.1c02613. Epub 2021 Nov 22. Inorg Chem. 2022. PMID: 34807595 Free PMC article.
-
Novel Pt-Ag3PO4/CdS/Chitosan Nanocomposite with Enhanced Photocatalytic and Biological Activities.Nanomaterials (Basel). 2020 Nov 23;10(11):2320. doi: 10.3390/nano10112320. Nanomaterials (Basel). 2020. PMID: 33238536 Free PMC article.
-
Layered Molybdenum (Meta)phosphate for Photoreduction of Hexavalent Chromium and Degradation of Methylene Blue under Sunlight Radiance.ACS Omega. 2022 Jul 22;7(30):26632-26640. doi: 10.1021/acsomega.2c02824. eCollection 2022 Aug 2. ACS Omega. 2022. PMID: 35936433 Free PMC article.
-
Investigating the Reductive Phosphatization Reaction Pathway in the Synthesis of Transition Metal Phosphates: A Case Study on Titanium Phosphates.Inorg Chem. 2025 Feb 10;64(5):2425-2432. doi: 10.1021/acs.inorgchem.4c04776. Epub 2025 Jan 24. Inorg Chem. 2025. PMID: 39854175 Free PMC article.
References
-
- Hutchings G.J. Vanadium phosphate: A new look at the active components of catalysts for the oxidation of butane to maleic anhydride. J. Mater. Chem. 2004;14:3385–3395. doi: 10.1039/b404610m. - DOI
-
- Muneyama E., Kunishige A., Ohdan K., Ai M. Reduction and reoxidation of iron phosphate and its catalytic activity for oxidative dehydrogenation of isobutyric acid. J. Catal. 1996;158:378–384. doi: 10.1006/jcat.1996.0039. - DOI
-
- Wang Y., Wang X.X., Su Z., Guo Q., Tang Q.H., Zhang Q.H., Wan H.L. SBA-15-supported iron phosphate catalyst for partial oxidation of methane to formaldehyde. Catal. Today. 2004;93–95:155–161.
-
- Lin R.H., Ding Y.J., Gong L.F., Dong W.D., Wang J.H., Zhang T. Efficient and stable silica-supported iron phosphate catalysts for oxidative bromination of methane. J. Catal. 2010;272:65–73. doi: 10.1016/j.jcat.2010.03.011. - DOI
-
- Padhi A.K., Nanjundaswamy K.S., Masquelier C., Okada S., Goodenough J.B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J. Electrochem. Soc. 1997;144:1609–1613. doi: 10.1149/1.1837649. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

