Quantum Dots as Multifunctional Materials for Tumor Imaging and Therapy
- PMID: 28809320
- PMCID: PMC5452096
- DOI: 10.3390/ma6020483
Quantum Dots as Multifunctional Materials for Tumor Imaging and Therapy
Abstract
The rapidly developing field of quantum dots (QDs) provides researchers with more options for imaging modalities and therapeutic strategies. In recent years, QDs were widely used as multifunctional materials for tumor imaging and therapy due to their characteristic properties such as semiconductive, zero-dimension and strong fluorescence. Nevertheless, there still exist the challenges of employing these properties of QDs for clinical diagnosis and therapy. Herein, we briefly review the development, properties and applications of QDs in tumor imaging and therapy. Future perspectives in these areas are also proposed as well.
Keywords: fluorescence; probe; quantum dots; semiconductor; tumor imaging and therapy.
Figures



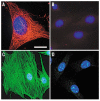
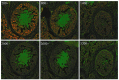





Similar articles
-
Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments.Nanomaterials (Basel). 2024 Jun 25;14(13):1088. doi: 10.3390/nano14131088. Nanomaterials (Basel). 2024. PMID: 38998693 Free PMC article. Review.
-
Quantum dot bioconjugates for in vitro diagnostics & in vivo imaging.Cancer Biomark. 2008;4(6):307-19. doi: 10.3233/cbm-2008-4603. Cancer Biomark. 2008. PMID: 19126959 Review.
-
Semiconductor quantum dots for biosensing and in vivo imaging.IEEE Trans Nanobioscience. 2009 Mar;8(1):4-12. doi: 10.1109/TNB.2009.2017321. Epub 2009 Mar 16. IEEE Trans Nanobioscience. 2009. PMID: 19304495 Review.
-
Quantum dots in cancer therapy.Expert Opin Drug Deliv. 2012 Jan;9(1):47-58. doi: 10.1517/17425247.2012.638624. Epub 2011 Dec 16. Expert Opin Drug Deliv. 2012. PMID: 22171712 Review.
-
Intracellular nucleic acid interactions facilitated by quantum dots: conceptualizing theranostics.Ther Deliv. 2012 Apr;3(4):479-99. doi: 10.4155/tde.12.15. Ther Deliv. 2012. PMID: 22834078 Review.
Cited by
-
Cellular distribution and cytotoxicity of graphene quantum dots with different functional groups.Nanoscale Res Lett. 2014 Mar 6;9(1):108. doi: 10.1186/1556-276X-9-108. Nanoscale Res Lett. 2014. PMID: 24597852 Free PMC article.
-
Application of functional quantum dot nanoparticles as fluorescence probes in cell labeling and tumor diagnostic imaging.Nanoscale Res Lett. 2015 Apr 10;10:171. doi: 10.1186/s11671-015-0873-8. eCollection 2015. Nanoscale Res Lett. 2015. PMID: 25897311 Free PMC article.
-
Interplay of Oxidative Stress, Inflammation, and Autophagy in RAW 264.7 Murine Macrophage Cell Line Challenged with Si/SiO2 Quantum Dots.Materials (Basel). 2023 Jul 19;16(14):5083. doi: 10.3390/ma16145083. Materials (Basel). 2023. PMID: 37512357 Free PMC article.
-
Gamma-Camera Direct Imaging of the Plasma and On/Intra Cellular Distribution of the 99mTc-DPD-Fe3O4 Dual-Modality Contrast Agent in Peripheral Human Blood.Materials (Basel). 2024 Jan 9;17(2):335. doi: 10.3390/ma17020335. Materials (Basel). 2024. PMID: 38255503 Free PMC article.
-
Nanocarrier-based drug combination therapy for glioblastoma.Theranostics. 2020 Jan 1;10(3):1355-1372. doi: 10.7150/thno.38147. eCollection 2020. Theranostics. 2020. PMID: 31938069 Free PMC article. Review.
References
-
- Hanson R., Kouwenhoven L.P., Petta J.R., Tarucha S., Vandersypen L.M.K. Spins in few-electron quantum dots. Rev. Mod. Phys. 2007;79:1217–1265. doi: 10.1103/RevModPhys.79.1217. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

