A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation
- PMID: 28809322
- PMCID: PMC5452089
- DOI: 10.3390/ma6020517
A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation
Abstract
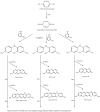
This review discusses a new aspect to the safety profile of oxidative hair dyes using data already in the public domain. These dyes contain secondary amines that are capable of forming potentially carcinogenic nitrosamine derivatives when exposed to atmospheric pollution. Numerous scientific articles confirm the existence of secondary amines in hair dyes (and their intermediates), the possibility of nitrosation by atmospheric NOx of secondary amines to give the N-nitrosamines, and the significant safety risks on N-nitrosamines. It is believed that such nitrosamine derivatives should be investigated more fully in the interests of consumer safety.
Keywords: N-nitroso compounds (NOC); cancer; hair dye; nitrosamine; p-phenylenediamine (PPD); risk.
Figures
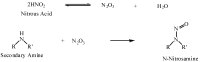

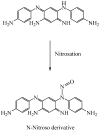
References
-
- Charle R., Sag G. Early synthetic organic hair dyes. Manuf. Chem. Aerosol News. 1967:33–37.
-
- Green A. Landmarks in the evolution of the dyestuff industry during the past half-century. J. Soc. Dyers Colour. 1934:49–64.
-
- European Commission Scientific Committee on Consumer Products (SCCP) Memorandum on Hair Dye Substances and Their Skin Sensitising Properties. [(accessed on 28 January 2013)]. Available online: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_s_05.pdf.
-
- European Commission Scientific Committee on Consumer Safety (SCCS) Opinion on Toluene-2,5-diamine and Its Sulphate. [(accessed on 28 January 2013)]. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sc....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

