Solubilization of Hydrophobic Dyes in Surfactant Solutions
- PMID: 28809328
- PMCID: PMC5452102
- DOI: 10.3390/ma6020580
Solubilization of Hydrophobic Dyes in Surfactant Solutions
Abstract
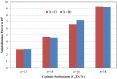
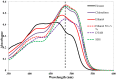
In this paper, the use of surfactants for solubilization of hydrophobic organic dyes (mainly solvent and disperse dyes) has been reviewed. The effect of parameters such as the chemical structures of the surfactant and the dye, addition of salt and of polyelectrolytes, pH, and temperature on dye solubilization has been discussed. Surfactant self-assemble into micelles in aqueous solution and below the concentration where this occurs-the critical micelle concentration (CMC)-there is no solubilization. Above the CMC, the amount of solubilized dye increases linearly with the increase in surfactant concentration. It is demonstrated that different surfactants work best for different dyes. In general, nonionic surfactants have higher solubilization power than anionic and cationic surfactants. It is likely that the reason for the good performance of nonionic surfactants is that they allow dyes to be accommodated not only in the inner, hydrocarbon part of the micelle but also in the headgroup shell. It is demonstrated that the location of a dye in a surfactant micelle can be assessed from the absorption spectrum of the dye-containing micellar solution.
Keywords: dye; hydrophobic; micelle; solubilization; surfactant; water insoluble.
Figures













References
-
- Mcbain J.W., Johnson K.E. Solubilization and the colloidal micelles in soap solution. J. Am. Chem. Soc. 1944;66:9–13. doi: 10.1021/ja01229a004. - DOI
-
- McBain J.W., Merrill R.C., Vinograd J.R. The solubilization of water-insoluble dye in dilute solutions of aqueous detergents. J. Am. Chem. Soc. 1941;63:670–676. doi: 10.1021/ja01848a011. - DOI
-
- Merrill R.C., Mcbain J.W. Studies on solubilization. J. Phys. Chem. 1942;46:10–19. doi: 10.1021/j150415a002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

