"Smart" Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications
- PMID: 28809338
- PMCID: PMC5512797
- DOI: 10.3390/ma6030738
"Smart" Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications
Abstract
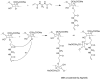
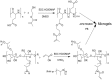
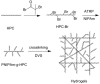
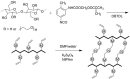
Cellulose is the most abundant biomass material in nature, and possesses some promising properties, such as mechanical robustness, hydrophilicity, biocompatibility, and biodegradability. Thus, cellulose has been widely applied in many fields. "Smart" materials based on cellulose have great advantages-especially their intelligent behaviors in reaction to environmental stimuli-and they can be applied to many circumstances, especially as biomaterials. This review aims to present the developments of "smart" materials based on cellulose in the last decade, including the preparations, properties, and applications of these materials. The preparations of "smart" materials based on cellulose by chemical modifications and physical incorporating/blending were reviewed. The responsiveness to pH, temperature, light, electricity, magnetic fields, and mechanical forces, etc. of these "smart" materials in their different forms such as copolymers, nanoparticles, gels, and membranes were also reviewed, and the applications as drug delivery systems, hydrogels, electronic active papers, sensors, shape memory materials and smart membranes, etc. were also described in this review.
Keywords: cellulose; drug delivery; smart materials; stimuli-responsive.
Figures







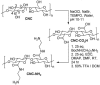
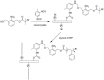



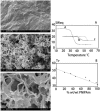
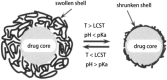




References
-
- Doelker E. Cellulose derivatives. Adv. Polym. Sci. 1993;107:199–265.
-
- Klemm D., Philipp B., Heinze T., Heinze U., Wagenknecht W. Comprehensive Cellulose Chemistry, Volume 1: Fundamentals and Analytical Methods. WILEY-VCH Verlag GmbH; Weinheim, Germany: 1998.
-
- Bledzki A.K., Gassan J. Composites reinforced with cellulose based fibers. Progr. Polym. Sci. 1999;24:221–274. doi: 10.1016/S0079-6700(98)00018-5. - DOI
-
- Kalia S., Kaith B.S., Kaur I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009;49:1253–1272. doi: 10.1002/pen.21328. - DOI
-
- Mashkour M., Tajvidi M., Kimura T., Kimura F., Ebrahimi G. Fabricating unidirectional magnetic papers using permanent magnets to align magnetic nanoparticale coveres natural cellulose fibers. BioResources. 2011;6:4731–4738.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

