Evidence for newly generated interneurons in the basolateral amygdala of adult mice
- PMID: 28809399
- PMCID: PMC5822453
- DOI: 10.1038/mp.2017.134
Evidence for newly generated interneurons in the basolateral amygdala of adult mice
Abstract
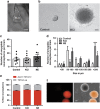
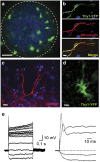
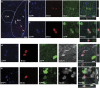
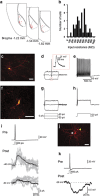
New neurons are continually generated from the resident populations of precursor cells in selective niches of the adult mammalian brain such as the hippocampal dentate gyrus and the olfactory bulb. However, whether such cells are present in the adult amygdala, and their neurogenic capacity, is not known. Using the neurosphere assay, we demonstrate that a small number of precursor cells, the majority of which express Achaete-scute complex homolog 1 (Ascl1), are present in the basolateral amygdala (BLA) of the adult mouse. Using neuron-specific Thy1-YFP transgenic mice, we show that YFP+ cells in BLA-derived neurospheres have a neuronal morphology, co-express the neuronal marker βIII-tubulin, and generate action potentials, confirming their neuronal phenotype. In vivo, we demonstrate the presence of newly generated BrdU-labeled cells in the adult BLA, and show that a proportion of these cells co-express the immature neuronal marker doublecortin (DCX). Furthermore, we reveal that a significant proportion of GFP+ neurons (~23%) in the BLA are newly generated (BrdU+) in DCX-GFP mice, and using whole-cell recordings in acute slices we demonstrate that the GFP+ cells display electrophysiological properties that are characteristic of interneurons. Using retrovirus-GFP labeling as well as the Ascl1CreERT2 mouse line, we further confirm that the precursor cells within the BLA give rise to mature and functional interneurons that persist in the BLA for at least 8 weeks after their birth. Contextual fear conditioning has no effect on the number of neurospheres or BrdU-labeled cells in the BLA, but produces an increase in hippocampal cell proliferation. These results demonstrate that neurogenic precursor cells are present in the adult BLA, and generate functional interneurons, but also show that their activity is not regulated by an amygdala-dependent learning paradigm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005; 28: 223–250. - PubMed
-
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 2008; 11: 1153–1161. - PubMed
-
- Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature 2001; 412: 736–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

