CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide
- PMID: 28809766
- PMCID: PMC6362827
- DOI: 10.1097/j.pain.0000000000001002
CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide
Abstract
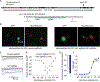
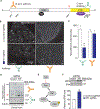
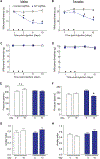
Neurofibromatosis type 1 (NF1) is a rare autosomal dominant disease linked to mutations of the Nf1 gene. Patients with NF1 commonly experience severe pain. Studies on mice with Nf1 haploinsufficiency have been instructive in identifying sensitization of ion channels as a possible cause underlying the heightened pain suffered by patients with NF1. However, behavioral assessments of Nf1 mice have led to uncertain conclusions about the potential causal role of Nf1 in pain. We used the clustered regularly interspaced short palindromic repeats (CRISPR)-associated 9 (CRISPR/Cas9) genome editing system to create and mechanistically characterize a novel rat model of NF1-related pain. Targeted intrathecal delivery of guide RNA/Cas9 nuclease plasmid in combination with a cationic polymer was used to generate allele-specific C-terminal truncation of neurofibromin, the protein encoded by the Nf1 gene. Rats with truncation of neurofibromin, showed increases in voltage-gated calcium (specifically N-type or CaV2.2) and voltage-gated sodium (particularly tetrodotoxin-sensitive) currents in dorsal root ganglion neurons. These gains-of-function resulted in increased nociceptor excitability and behavioral hyperalgesia. The cytosolic regulatory protein collapsin response mediator protein 2 (CRMP2) regulates activity of these channels, and also binds to the targeted C-terminus of neurofibromin in a tripartite complex, suggesting a possible mechanism underlying NF1 pain. Prevention of CRMP2 phosphorylation with (S)-lacosamide resulted in normalization of channel current densities, excitability, as well as of hyperalgesia following CRISPR/Cas9 truncation of neurofibromin. These studies reveal the protein partners that drive NF1 pain and suggest that CRMP2 is a key target for therapeutic intervention.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

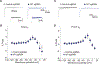




References
-
- Bicudo NP, de Menezes Neto BF, da Silva de Avo LR, Germano CM, Melo DG Quality of life in adults with neurofibromatosis 1 in Brazil. J Genet Couns 2016;25:1063–74. - PubMed
-
- Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(1) channel complex. Nat Med 2011;17: 822–9. - PMC - PubMed
-
- Brittain JM, Wang Y, Eruvwetere O, Khanna R. Cdk5-mediated phosphorylation of CRMP-2 enhances its interaction with CaV2.2. FEBS Lett 2012;586:3813–18. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

