Impact of fortified versus unfortified lipid-based supplements on morbidity and nutritional status: A randomised double-blind placebo-controlled trial in ill Gambian children
- PMID: 28809926
- PMCID: PMC5557358
- DOI: 10.1371/journal.pmed.1002377
Impact of fortified versus unfortified lipid-based supplements on morbidity and nutritional status: A randomised double-blind placebo-controlled trial in ill Gambian children
Abstract
Background: Multiple micronutrients (MMN) are commonly prescribed in pediatric primary healthcare in sub-Saharan Africa to improve nutritional status and appetite without evidence for their effectiveness or international clinical guidelines. Community-wide MMN supplementation has shown limited and heterogeneous impact on growth and morbidity. Short-term ready-to-use therapeutic foods in acutely sick children in a hospital setting also had limited efficacy regarding subsequent growth. The effectiveness of MMN in improving morbidity or growth in sick children presenting for primary care has not been assessed.
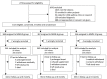
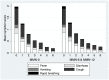
Methods and findings: We undertook a double-blind randomised controlled trial of small-quantity lipid-based nutrient supplements (SQ-LNS) fortified with 23 micronutrients in children aged 6 months (mo) to 5 years (y) presenting with an illness at a rural primary healthcare centre in The Gambia. Primary outcomes were repeat clinic presentations and growth over 24 wk. Participants were randomly assigned to receive 1 of 3 interventions: (1) supplementation with micronutrient-fortified SQ-LNS for 12 wk (MMN-12), (2) supplementation with micronutrient-fortified SQ-LNS for 6 wk followed by unfortified SQ-LNS for 6 wk (MMN-6), or (3) supplementation with unfortified SQ-LNS for 12 wk (MMN-0) to be consumed in daily portions. Treatment masking used 16 letters per 6-wk block in the randomisation process. Blinded intention-to-treat analysis based on a prespecified statistical analysis plan included all participants eligible and correctly enrolled. Between December 2009 and June 2011, 1,101 children (age 6-60 mo, mean 25.5 mo) were enrolled, and 1,085 were assessed (MMN-0 = 361, MMN-6 = 362, MMN-12 = 362). MMN supplementation was associated with a small increase in height-for-age z-scores 24 wk after recruitment (effect size for MMN groups combined: 0.084 SD/24 wk, 95% CI: 0.005, 0.168; p = 0.037; equivalent to 2-5 mm depending on age). No significant difference in frequency of morbidity measured by the number of visits to the clinic within 24 wk follow-up was detected with 0.09 presentations per wk for all groups (MMN-0 versus MMN-6: adjusted incidence rate ratio [IRR] 1.03, 95% CI: 0.92, 1.16; MMN-0 versus MMN-12: 1.05, 95% CI: 0.93, 1.18). In post hoc analysis, clinic visits significantly increased by 43% over the first 3 wk of fortified versus unfortified SQ-LNS (adjusted IRR 1.43; 95% CI: 1.07, 1.92; p = 0.016), with respiratory presentations increasing by 52% with fortified SQ-LNS (adjusted IRR 1.52; 95% CI: 1.01, 2.30; p = 0.046). The number of severe adverse events during supplementation were similar between groups (MMN-0 = 20 [1 death]; MMN-6 = 21 [1 death]; MMN-12 = 20 [0 death]). No participant withdrew due to adverse effects. Study limitations included the lack of supervision of daily supplementation.
Conclusion: Prescribing micronutrient-fortified SQ-LNS to ill children presenting for primary care in rural Gambia had a very small effect on linear growth and did not reduce morbidity compared to unfortified SQ-LNS. An early increase in repeat visits indicates a need for the establishment of evidence-based guidelines and caution with systematic prescribing of MMN. Future research should be directed at understanding the mechanisms behind the lack of effect of MMN supplementation on morbidity measures and limited effect on growth.
Trial registration: ISRCTN 73571031.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382(9890): 427–51. doi: 10.1016/S0140-6736(13)60937-X - DOI - PubMed
-
- Risk R, Naismith H, Burnett A, Moore SE, Cham M, Unger S. Rational prescribing in paediatrics in a resource-limited setting. Archives of disease in childhood 2013; 98(7): 503–9. doi: 10.1136/archdischild-2012-302987 - DOI - PubMed
-
- Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr 2007; 86(2): 412–20. - PubMed
-
- Hess SY, Abbeddou S, Jimenez EY, Some JW, Vosti SA, Quedraogo ZP et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and Wasting in young burkinabe children: A cluster-randomized trial. PLoS ONE 2015; 10 (3) (0122242). - PMC - PubMed
-
- Maleta KM, Phuka J, Alho L, Cheung YB, Dewey KG, Ashorn U et al. Provision of 10–40 g/d Lipid-Based Nutrient Supplements from 6 to 18 Months of Age Does Not Prevent Linear Growth Faltering in Malawi. J Nutr 2015; 145(8): 1909–15. doi: 10.3945/jn.114.208181 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical