Structure-function analyses of metal-binding sites of HypA reveal residues important for hydrogenase maturation in Helicobacter pylori
- PMID: 28809946
- PMCID: PMC5557546
- DOI: 10.1371/journal.pone.0183260
Structure-function analyses of metal-binding sites of HypA reveal residues important for hydrogenase maturation in Helicobacter pylori
Abstract
The nickel-containing enzymes of Helicobacter pylori, urease and hydrogenase, are essential for efficient colonization in the human stomach. The insertion of nickel into urease and hydrogenase is mediated by the accessory protein HypA. HypA contains an N-terminal nickel-binding site and a dynamic structural zinc-binding site. The coordination of nickel and zinc within HypA is known to be critical for urease maturation and activity. Herein, we test the hydrogenase activity of a panel of H. pylori mutant strains containing point mutations within the nickel- and zinc-binding sites. We found that the residues that are important for hydrogenase activity are those that were similarly vital for urease activity. Thus, the zinc and metal coordination sites of HypA play similar roles in urease and hydrogenase maturation. In other pathogenic bacteria, deletion of hydrogenase leads to a loss in acid resistance. Thus, the acid resistance of two strains of H. pylori containing a hydrogenase deletion was also tested. These mutant strains demonstrated wild-type levels of acid resistance, suggesting that in H. pylori, hydrogenase does not play a role in acid resistance.
Conflict of interest statement
Figures



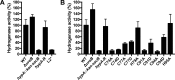
Similar articles
-
Dynamic HypA zinc site is essential for acid viability and proper urease maturation in Helicobacter pylori.Metallomics. 2015 Apr;7(4):674-82. doi: 10.1039/c4mt00306c. Metallomics. 2015. PMID: 25608738 Free PMC article.
-
Requirement of nickel metabolism proteins HypA and HypB for full activity of both hydrogenase and urease in Helicobacter pylori.Mol Microbiol. 2001 Jan;39(1):176-82. doi: 10.1046/j.1365-2958.2001.02244.x. Mol Microbiol. 2001. PMID: 11123699
-
Characterization of Helicobacter pylori nickel metabolism accessory proteins needed for maturation of both urease and hydrogenase.J Bacteriol. 2003 Feb;185(3):726-34. doi: 10.1128/JB.185.3.726-734.2003. J Bacteriol. 2003. PMID: 12533448 Free PMC article.
-
Moving nickel along the hydrogenase-urease maturation pathway.Metallomics. 2022 May 13;14(5):mfac003. doi: 10.1093/mtomcs/mfac003. Metallomics. 2022. PMID: 35556134 Review.
-
Common themes and unique proteins for the uptake and trafficking of nickel, a metal essential for the virulence of Helicobacter pylori.Front Cell Infect Microbiol. 2013 Dec 9;3:94. doi: 10.3389/fcimb.2013.00094. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24367767 Free PMC article. Review.
Cited by
-
Edwardsiella tarda Sip2: A Serum-Induced Protein That Is Essential to Serum Survival, Acid Resistance, Intracellular Replication, and Host Infection.Front Microbiol. 2018 May 25;9:1084. doi: 10.3389/fmicb.2018.01084. eCollection 2018. Front Microbiol. 2018. PMID: 29887847 Free PMC article.
-
Nickel Metalloregulators and Chaperones.Inorganics (Basel). 2019 Aug;7(8):10.3390/inorganics7080104. doi: 10.3390/inorganics7080104. Epub 2019 Aug 19. Inorganics (Basel). 2019. PMID: 32133383 Free PMC article.
-
Chronic Diarrhea Due to Aeromonas hydrophila in an Immunosuppressed Patient with a Pancreas-Kidney Transplant.Pathogens. 2023 Sep 11;12(9):1151. doi: 10.3390/pathogens12091151. Pathogens. 2023. PMID: 37764959 Free PMC article.
-
Evodiamine Inhibits Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation.Int J Mol Sci. 2021 Mar 25;22(7):3385. doi: 10.3390/ijms22073385. Int J Mol Sci. 2021. PMID: 33806161 Free PMC article.
-
Zerumbone Inhibits Helicobacter pylori Urease Activity.Molecules. 2021 May 1;26(9):2663. doi: 10.3390/molecules26092663. Molecules. 2021. PMID: 34062878 Free PMC article.
References
-
- Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284(5418):1328–33. . - PubMed
-
- International Agency for Research on Cancer. A review of human carcinogens. Part B: Biological agents. Lyons, France: International Agency for Research on Cancer; 2012.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

