The transcriptional program, functional heterogeneity, and clinical targeting of mast cells
- PMID: 28811324
- PMCID: PMC5584128
- DOI: 10.1084/jem.20170910
The transcriptional program, functional heterogeneity, and clinical targeting of mast cells
Abstract
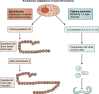
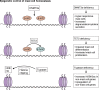
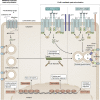
Mast cells are unique tissue-resident immune cells that express an array of receptors that can be activated by several extracellular cues, including antigen-immunoglobulin E (IgE) complexes, bacteria, viruses, cytokines, hormones, peptides, and drugs. Mast cells constitute a small population in tissues, but their extraordinary ability to respond rapidly by releasing granule-stored and newly made mediators underpins their importance in health and disease. In this review, we document the biology of mast cells and introduce new concepts and opinions regarding their role in human diseases beyond IgE-mediated allergic responses and antiparasitic functions. We bring to light recent discoveries and developments in mast cell research, including regulation of mast cell functions, differentiation, survival, and novel mouse models. Finally, we highlight the current and future opportunities for therapeutic intervention of mast cell functions in inflammatory diseases.
© 2017 Cildir et al.
Figures