Inhibitors of the Histone Methyltransferases EZH2/1 Induce a Potent Antiviral State and Suppress Infection by Diverse Viral Pathogens
- PMID: 28811345
- PMCID: PMC5559635
- DOI: 10.1128/mBio.01141-17
Inhibitors of the Histone Methyltransferases EZH2/1 Induce a Potent Antiviral State and Suppress Infection by Diverse Viral Pathogens
Abstract
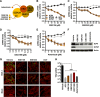
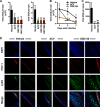
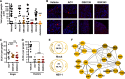
Epigenetic regulation is based on a network of complexes that modulate the chromatin character and structure of the genome to impact gene expression, cell fate, and development. Thus, epigenetic modulators represent novel therapeutic targets used to treat a range of diseases, including malignancies. Infectious pathogens such as herpesviruses are also regulated by cellular epigenetic machinery, and epigenetic therapeutics represent a novel approach used to control infection, persistence, and the resulting recurrent disease. The histone H3K27 methyltransferases EZH2 and EZH1 (EZH2/1) are epigenetic repressors that suppress gene transcription via propagation of repressive H3K27me3-enriched chromatin domains. However, while EZH2/1 are implicated in the repression of herpesviral gene expression, inhibitors of these enzymes suppressed primary herpes simplex virus (HSV) infection in vitro and in vivo Furthermore, these compounds blocked lytic viral replication following induction of HSV reactivation in latently infected sensory ganglia. Suppression correlated with the induction of multiple inflammatory, stress, and antipathogen pathways, as well as enhanced recruitment of immune cells to in vivo infection sites. Importantly, EZH2/1 inhibitors induced a cellular antiviral state that also suppressed infection with DNA (human cytomegalovirus, adenovirus) and RNA (Zika virus) viruses. Thus, EZH2/1 inhibitors have considerable potential as general antivirals through the activation of cellular antiviral and immune responses.IMPORTANCE A significant proportion of the world's population is infected with herpes simplex virus. Primary infection and subsequent recurrent reactivation can result in diseases ranging from mild lesions to severe ocular or neurological damage. Herpesviruses are subject to epigenetic regulation that modulates viral gene expression, lytic replication, and latency-reactivation cycles. Thus, epigenetic pharmaceuticals have the potential to alter the course of infection and disease. Here, while the histone methyltransferases EZH2/1 are implicated in the suppression of herpesviruses, inhibitors of these repressors unexpectedly suppress viral infection in vitro and in vivo by induction of key components of cellular innate defense pathways. These inhibitors suppress infection by multiple viral pathogens, indicating their potential as broad-spectrum antivirals.
Keywords: Zika virus; antiviral; chromatin; epigenetics; herpesvirus; immune mechanisms; innate immunity.
Figures



References
-
- Whitley RJ, Kimberlin DW, Prober CG. 2007. Pathogenesis and disease, p 589–601. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, New York, NY. - PubMed
-
- Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 15:886–892. doi: 10.1038/nm.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
