The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis
- PMID: 28811522
- PMCID: PMC5557893
- DOI: 10.1038/s41598-017-08225-1
The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis
Erratum in
-
Author Correction: The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis.Sci Rep. 2021 Jun 17;11(1):13132. doi: 10.1038/s41598-021-91474-y. Sci Rep. 2021. PMID: 34140534 Free PMC article. No abstract available.
Abstract
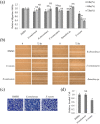
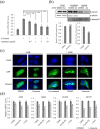
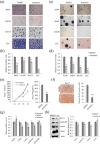
Lichens are symbiotic organisms that produce various secondary metabolites. Here, different lichen extracts were examined to identify secondary metabolites with anti-migratory activity against human lung cancer cells. Everniastrum vexans had the most potent inhibitory activity, and atranorin was identified as an active subcomponent of this extract. Atranorin suppressed β-catenin-mediated TOPFLASH activity by inhibiting the nuclear import of β-catenin and downregulating β-catenin/LEF and c-jun/AP-1 downstream target genes such as CD44, cyclin-D1 and c-myc. Atranorin decreased KAI1 C-terminal interacting tetraspanin (KITENIN)-mediated AP-1 activity and the activity of the KITENIN 3'-untranslated region. The nuclear distribution of the AP-1 transcriptional factor, including c-jun and c-fos, was suppressed in atranorin-treated cells, and atranorin inhibited the activity of Rho GTPases including Rac1, Cdc42, and RhoA, whereas it had no effect on epithelial-mesenchymal transition markers. STAT-luciferase activity and nuclear STAT levels were decreased, whereas total STAT levels were moderately reduced. The human cell motility and lung cancer RT² Profiler PCR Arrays identified additional atranorin target genes. Atranorin significantly inhibited tumorigenesis in vitro and in vivo. Taken together, our results indicated that E. vexans and its subcomponent atranorin may inhibit lung cancer cell motility and tumorigenesis by affecting AP-1, Wnt, and STAT signaling and suppressing RhoGTPase activity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA: a cancer journal for clinicians. 2011;61:91–112. - PubMed
-
- Nash III, T. H. Lichen sensitivity to air pollution. Lichen biology2 (2008).
-
- Elix J, Stocker-Wörgötter E. Biochemistry and secondary metabolites. Lichen biology. 1996;1:154–180.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

