Totipotency segregates between the sister blastomeres of two-cell stage mouse embryos
- PMID: 28811525
- PMCID: PMC5557898
- DOI: 10.1038/s41598-017-08266-6
Totipotency segregates between the sister blastomeres of two-cell stage mouse embryos
Abstract
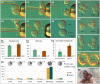
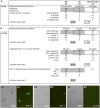
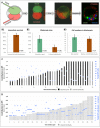
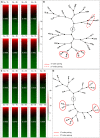
Following fertilization in mammals, it is generally accepted that totipotent cells are exclusive to the zygote and to each of the two blastomeres originating from the first mitotic division. This model of totipotency was inferred from a minority of cases in which blastomeres produced monozygotic twins in mice. Was this due to experimental limitation or biological constraint? Here we removed experimental obstacles and achieved reliable quantification of the prevalence of dual totipotency among mouse two-cell stage blastomeres. We separated the blastomeres of 1,252 two-cell embryos, preserving 1,210 of the pairs. Two classes of monozygotic twins became apparent at the blastocyst stage: 27% formed a functional epiblast in both members (concordant), and 73% did so in only one member of the pair (discordant) - a partition that proved insensitive to oocyte quality, sperm-entry point, culture environment and pattern of cleavage. In intact two-cell embryos, the ability of sister blastomeres to generate epiblast was also skewed. Class discovery clustering of the individual blastomeres' and blastocysts' transcriptomes points to an innate origin of concordance and discordance rather than developmental acquisition. Our data place constraints on the commonly accepted idea that totipotency is allocated equally between the two-cell stage blastomeres in mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Retrospective analysis: reproducibility of interblastomere differences of mRNA expression in 2-cell stage mouse embryos is remarkably poor due to combinatorial mechanisms of blastomere diversification.Mol Hum Reprod. 2018 Jul 1;24(7):388-400. doi: 10.1093/molehr/gay021. Mol Hum Reprod. 2018. PMID: 29746690
-
Totipotency of mouse zygotes extends to single blastomeres of embryos at the four-cell stage.Sci Rep. 2021 May 27;11(1):11167. doi: 10.1038/s41598-021-90653-1. Sci Rep. 2021. PMID: 34045607 Free PMC article.
-
Differences in blastomere totipotency in 2-cell mouse embryos are a maternal trait mediated by asymmetric mRNA distribution.Mol Hum Reprod. 2019 Nov 30;25(11):729-744. doi: 10.1093/molehr/gaz051. Mol Hum Reprod. 2019. PMID: 31504820 Free PMC article.
-
The Principal Forces of Oocyte Polarity Are Evolutionary Conserved but May Not Affect the Contribution of the First Two Blastomeres to the Blastocyst Development in Mammals.PLoS One. 2016 Mar 31;11(3):e0148382. doi: 10.1371/journal.pone.0148382. eCollection 2016. PLoS One. 2016. PMID: 27030988 Free PMC article.
-
Totipotency continuity from zygote to early blastomeres: a model under revision.Reproduction. 2019 Aug;158(2):R49-R65. doi: 10.1530/REP-18-0462. Reproduction. 2019. PMID: 30978695 Review.
Cited by
-
Totipotency or plenipotency: rethinking stem cell bipotentiality.Curr Opin Genet Dev. 2025 Jun;92:102342. doi: 10.1016/j.gde.2025.102342. Epub 2025 Mar 19. Curr Opin Genet Dev. 2025. PMID: 40107116 Free PMC article. Review.
-
The effects of embryo splitting on Cdx2, Sox2, Oct4, and Nanog gene expression in mouse blastocysts.Iran J Vet Res. 2022;23(4):331-336. doi: 10.22099/IJVR.2022.42487.6172. Iran J Vet Res. 2022. PMID: 36874188 Free PMC article.
-
On transposons and totipotency.Philos Trans R Soc Lond B Biol Sci. 2020 Mar 30;375(1795):20190339. doi: 10.1098/rstb.2019.0339. Epub 2020 Feb 10. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32075562 Free PMC article.
-
Building Pluripotency Identity in the Early Embryo and Derived Stem Cells.Cells. 2021 Aug 10;10(8):2049. doi: 10.3390/cells10082049. Cells. 2021. PMID: 34440818 Free PMC article. Review.
-
Male gamete copies to characterize genome inheritance and generate progenies.Sci Rep. 2025 May 4;15(1):15600. doi: 10.1038/s41598-025-99188-1. Sci Rep. 2025. PMID: 40320458 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

