Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity
- PMID: 28812548
- PMCID: PMC5815964
- DOI: 10.1038/mi.2017.55
Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity
Abstract
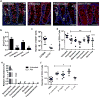
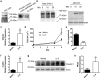
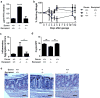
Interactions between the microbiota and distal gut are important for the maintenance of a healthy intestinal barrier; dysbiosis of intestinal microbial communities has emerged as a likely contributor to diseases that arise at the level of the mucosa. Intraepithelial lymphocytes (IELs) are positioned within the epithelial barrier, and in the small intestine they function to maintain epithelial homeostasis. We hypothesized that colon IELs promote epithelial barrier function through the expression of cytokines in response to interactions with commensal bacteria. Profiling of bacterial 16S ribosomal RNA revealed that candidate bacteria in the order Bacteroidales are sufficient to promote IEL presence in the colon that in turn produce interleukin-6 (IL-6) in a MyD88 (myeloid differentiation primary response 88)-dependent manner. IEL-derived IL-6 is functionally important in the maintenance of the epithelial barrier as IL-6-/- mice were noted to have increased paracellular permeability, decreased claudin-1 expression, and a thinner mucus gel layer, all of which were reversed by transfer of IL-6+/+ IELs, leading to protection of mice in response to Citrobacter rodentium infection. Therefore, we conclude that microbiota provide a homeostatic role for epithelial barrier function through regulation of IEL-derived IL-6.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures



References
-
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature reviews. Immunology. 2010;10:159–169. - PubMed
-
- Yu Q, et al. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. Journal of immunology. 2006;176:6180–6185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

