DESIGNER Extracts as Tools to Balance Estrogenic and Chemopreventive Activities of Botanicals for Women's Health
- PMID: 28812892
- PMCID: PMC5765536
- DOI: 10.1021/acs.jnatprod.7b00284
DESIGNER Extracts as Tools to Balance Estrogenic and Chemopreventive Activities of Botanicals for Women's Health
Abstract
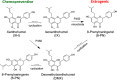
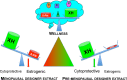
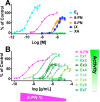
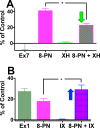
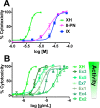
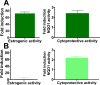
Botanical dietary supplements contain multiple bioactive compounds that target numerous biological pathways. The lack of uniform standardization requirements is one reason that inconsistent clinical effects are reported frequently. The multifaceted biological interactions of active principles can be disentangled by a coupled pharmacological/phytochemical approach using specialized ("knock-out") extracts. This is demonstrated for hops, a botanical for menopausal symptom management. Employing targeted, adsorbent-free countercurrent separation, Humulus lupulus extracts were designed for pre- and postmenopausal women by containing various amounts of the phytoestrogen 8-prenylnaringenin (8-PN) and the chemopreventive constituent xanthohumol (XH). Analysis of their estrogenic (alkaline phosphatase), chemopreventive (NAD(P)H-quinone oxidoreductase 1 [NQO1]), and cytotoxic bioactivities revealed that the estrogenicity of hops is a function of 8-PN, whereas their NQO1 induction and cytotoxic properties depend on XH levels. Antagonization of the estrogenicity of 8-PN by elevated XH concentrations provided evidence for the interdependence of the biological effects. A designed postmenopausal hop extract was prepared to balance 8-PN and XH levels for both estrogenic and chemopreventive properties. An extract designed for premenopausal women contains reduced 8-PN levels and high XH concentrations to minimize estrogenic while retaining chemopreventive properties. This study demonstrates the feasibility of modulating the concentrations of bioactive compounds in botanical extracts for potentially improved efficacy and safety.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.B. is director of research and development/analytics of Hopsteiner HHV GmbH. The other authors declare no competing financial interest.
Figures

References
-
- Smith T.; Lynch M. E.; Johnson J.; Kawa K.; Bauman H.; Blumenthal M. Herbalgram 2015, 107, 52–59.
-
- Cordell G. A. Phytochem. Lett. 2015, 11, 332–346. 10.1016/j.phytol.2014.09.003. - DOI
-
- Global Industry Analysts, I. Herbal Supplements and Remedies: A Global, Strategic Business Report. http://www.strategyr.com/MarketResearch/Herbal_Supplements_and_Remedies_... (June 26, 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

