IgG cooperativity - Is there allostery? Implications for antibody functions and therapeutic antibody development
- PMID: 28812955
- PMCID: PMC5680800
- DOI: 10.1080/19420862.2017.1367074
IgG cooperativity - Is there allostery? Implications for antibody functions and therapeutic antibody development
Abstract
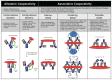
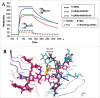
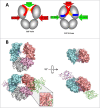
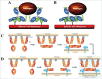
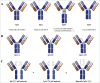
A central dogma in immunology is that an antibody's in vivo functionality is mediated by 2 independent events: antigen binding by the variable (V) region, followed by effector activation by the constant (C) region. However, this view has recently been challenged by reports suggesting allostery exists between the 2 regions, triggered by conformational changes or configurational differences. The possibility of allosteric signals propagating through the IgG domains complicates our understanding of the antibody structure-function relationship, and challenges the current subclass selection process in therapeutic antibody design. Here we review the types of cooperativity in IgG molecules by examining evidence for and against allosteric cooperativity in both Fab and Fc domains and the characteristics of associative cooperativity in effector system activation. We investigate the origin and the mechanism of allostery with an emphasis on the C-region-mediated effects on both V and C region interactions, and discuss its implications in biological functions. While available research does not support the existence of antigen-induced conformational allosteric cooperativity in IgGs, there is substantial evidence for configurational allostery due to glycosylation and sequence variations.
Keywords: IgG allostery; IgG subclass selection; antibody discovery; cooperativity; glycosylation; intermolecular interaction; intramolecular interaction; molecular engineering; structure and function.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
