Recent Advances in Electrospun Nanofiber Interfaces for Biosensing Devices
- PMID: 28813013
- PMCID: PMC5579928
- DOI: 10.3390/s17081887
Recent Advances in Electrospun Nanofiber Interfaces for Biosensing Devices
Abstract
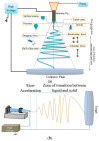
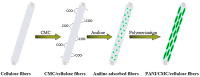
Electrospinning has emerged as a very powerful method combining efficiency, versatility and low cost to elaborate scalable ordered and complex nanofibrous assemblies from a rich variety of polymers. Electrospun nanofibers have demonstrated high potential for a wide spectrum of applications, including drug delivery, tissue engineering, energy conversion and storage, or physical and chemical sensors. The number of works related to biosensing devices integrating electrospun nanofibers has also increased substantially over the last decade. This review provides an overview of the current research activities and new trends in the field. Retaining the bioreceptor functionality is one of the main challenges associated with the production of nanofiber-based biosensing interfaces. The bioreceptors can be immobilized using various strategies, depending on the physical and chemical characteristics of both bioreceptors and nanofiber scaffolds, and on their interfacial interactions. The production of nanobiocomposites constituted by carbon, metal oxide or polymer electrospun nanofibers integrating bioreceptors and conductive nanomaterials (e.g., carbon nanotubes, metal nanoparticles) has been one of the major trends in the last few years. The use of electrospun nanofibers in ELISA-type bioassays, lab-on-a-chip and paper-based point-of-care devices is also highly promising. After a short and general description of electrospinning process, the different strategies to produce electrospun nanofiber biosensing interfaces are discussed.
Keywords: bioreceptor immobilization; biosensing devices; carbon nanofibers; carbon nanotubes; electrospinning; metal nanoparticles; metal oxide nanofibers; polymer nanofibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Lopez G.A., Estevez M.-C., Solera M., Lechuga L.M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonics. 2017;6:123–136. doi: 10.1515/nanoph-2016-0101. - DOI
-
- Kim J., Kumar R., Bandodkar A.J., Wang J. Advanced materials for printed wearable electrochemical devices: A review. Adv. Electron. Mater. 2017;3:1–15. doi: 10.1002/aelm.201600260. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

