The Helicobacter pylori type IV secretion system promotes IL-8 synthesis in a model of pediatric airway epithelium via p38 MAP kinase
- PMID: 28813514
- PMCID: PMC5557493
- DOI: 10.1371/journal.pone.0183324
The Helicobacter pylori type IV secretion system promotes IL-8 synthesis in a model of pediatric airway epithelium via p38 MAP kinase
Abstract
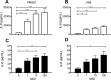
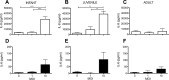
Epidemiologic studies have reported an inverse relationship between childhood Helicobacter pylori infection and development of allergic asthma. Because lung epithelium plays an important role in allergic asthma pathogenesis, we hypothesized that H. pylori may directly influence airway epithelial cell innate immune function, particularly in early childhood. To test our hypothesis, we established an in vitro H. pylori infection model using primary tracheobronchial epithelial cell cultures derived from infant, juvenile and adult rhesus monkeys. Airway epithelial cell cultures were infected with wild-type or cag pathogenicity island mutant H. pylori strains, followed by evaluation of IL-8 and IL-6 protein synthesis. We found that H. pylori primarily increased IL-8 synthesis in a MOI and age-dependent fashion, with a greater than 4-fold induction in infant versus adult cultures. H. pylori-induced IL-8 synthesis in infant and juvenile cultures was significantly reduced by cag pathogenicity island mutants, indicating a requirement for the type IV secretion system. Although peptidoglycan recognition of nucleotide binding oligomerization domain-containing protein 1 (NOD1) and NF-kappaB have been implicated as key cytokine signaling molecules for H. pylori infection in gastric epithelium, NOD1 (ML130) or NF-kappaB (JSH-23) inhibitors minimally affected IL-8 synthesis in airway epithelial cell cultures following H. pylori infection. In contrast, inhibition of the p38 MAP kinase pathway (SB203580) resulted in almost complete suppression of H. pylori-induced IL-8 synthesis. Collectively, these results indicate that H. pylori can preferentially elicit IL-8 synthesis in a model of pediatric airway epithelium using the type IV secretion system via p38 MAP kinase.
Conflict of interest statement
Figures




References
-
- Muller A, Oertli M, Arnold IC. H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell communication and signaling: CCS. 2011;9(1):25 Epub 2011/11/03. doi: 10.1186/1478-811x-9-25 ; PubMed Central PMCID: PMCPMC3214186. - DOI - PMC - PubMed
-
- Arnold IC, Hitzler I, Muller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Frontiers in cellular and infection microbiology. 2012;2:10 Epub 2012/08/25. doi: 10.3389/fcimb.2012.00010 ; PubMed Central PMCID: PMCPMC3417532. - DOI - PMC - PubMed
-
- Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198(4):553–60. Epub 2008/07/05. doi: 10.1086/590158 ; PubMed Central PMCID: PMC3902975. - DOI - PMC - PubMed
-
- Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167(8):821–7. Epub 2007/04/25. doi: 10.1001/archinte.167.8.821 . - DOI - PubMed
-
- Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121(8):3088–93. doi: 10.1172/JCI45041 ; PubMed Central PMCID: PMC3148731. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

