Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression
- PMID: 28813661
- PMCID: PMC5578409
- DOI: 10.1016/j.immuni.2017.07.014
Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression
Erratum in
-
Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression.Immunity. 2017 Sep 19;47(3):597. doi: 10.1016/j.immuni.2017.08.018. Immunity. 2017. PMID: 28930665 Free PMC article. No abstract available.
Abstract
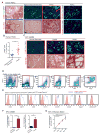
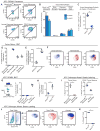
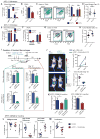
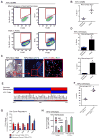
Tumor-associated macrophages (TAMs) are essential components of the cancer microenvironment and play critical roles in the regulation of tumor progression. Optimal therapeutic intervention requires in-depth understanding of the sources that sustain macrophages in malignant tissues. In this study, we investigated the ontogeny of TAMs in murine pancreatic ductal adenocarcinoma (PDAC) models. We identified both inflammatory monocytes and tissue-resident macrophages as sources of TAMs. Unexpectedly, significant portions of pancreas-resident macrophages originated from embryonic development and expanded through in situ proliferation during tumor progression. Whereas monocyte-derived TAMs played more potent roles in antigen presentation, embryonically derived TAMs exhibited a pro-fibrotic transcriptional profile, indicative of their role in producing and remodeling molecules in the extracellular matrix. Collectively, these findings uncover the heterogeneity of TAM origin and functions and could provide therapeutic insight for PDAC treatment.
Keywords: fibrosis; macrophage ontogeny; pancreas; pancreatic cancer; tissue-resident macrophage; tumor immunity.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
The Yolk Sac Feeds Pancreatic Tumors.Immunity. 2017 Aug 15;47(2):217-218. doi: 10.1016/j.immuni.2017.07.021. Immunity. 2017. PMID: 28813653
References
-
- Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–158. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 CA009621/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA203890/CA/NCI NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- DP1 DK109668/DK/NIDDK NIH HHS/United States
- K08 HL123519/HL/NHLBI NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 CA177670/CA/NCI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P50 CA196510/CA/NCI NIH HHS/United States
- R01 HL138466/HL/NHLBI NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

