Applied Swarm-based medicine: collecting decision trees for patterns of algorithms analysis
- PMID: 28814269
- PMCID: PMC5559810
- DOI: 10.1186/s12874-017-0400-y
Applied Swarm-based medicine: collecting decision trees for patterns of algorithms analysis
Abstract
Background: The objective consensus methodology has recently been applied in consensus finding in several studies on medical decision-making among clinical experts or guidelines. The main advantages of this method are an automated analysis and comparison of treatment algorithms of the participating centers which can be performed anonymously.
Methods: Based on the experience from completed consensus analyses, the main steps for the successful implementation of the objective consensus methodology were identified and discussed among the main investigators.
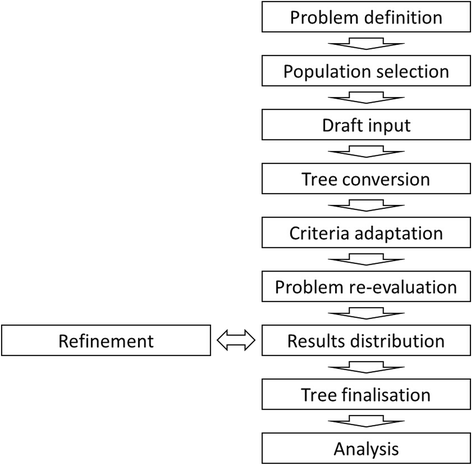
Results: The following steps for the successful collection and conversion of decision trees were identified and defined in detail: problem definition, population selection, draft input collection, tree conversion, criteria adaptation, problem re-evaluation, results distribution and refinement, tree finalisation, and analysis.
Conclusion: This manuscript provides information on the main steps for successful collection of decision trees and summarizes important aspects at each point of the analysis.
Keywords: Cancer; Consensus; Consensus finding; Decision tree; Radiotherapy; Swarm-based medicine.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

