Neurobiology of fear and specific phobias
- PMID: 28814472
- PMCID: PMC5580526
- DOI: 10.1101/lm.044115.116
Neurobiology of fear and specific phobias
Abstract
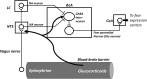
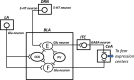
Fear, which can be expressed innately or after conditioning, is triggered when a danger or a stimulus predicting immediate danger is perceived. Its role is to prepare the body to face this danger. However, dysfunction in fear processing can lead to psychiatric disorders in which fear outweighs the danger or possibility of harm. Although recognized as highly debilitating, pathological fear remains insufficiently treated, indicating the importance of research on fear processing. The neurobiological basis of normal and pathological fear reactions is reviewed in this article. Innate and learned fear mechanisms, particularly those involving the amygdala, are considered. These fear mechanisms are also distinguished in specific phobias, which can indeed be nonexperiential (implicating innate, learning-independent mechanisms) or experiential (implicating learning-dependent mechanisms). Poor habituation and poor extinction are presented as dysfunctional mechanisms contributing to persistence of nonexperiential and experiential phobias, respectively.
© 2017 Garcia; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Adamec R, Bartoszyk GD, Burton P. 2004. Effects of systemic injections of Vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in rats. Eur J Pharmacol 504: 65–77. - PubMed
-
- Adamec R, Blundell J, Burton P. 2005. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol Behav 86: 75–91. - PubMed
-
- Aitken RC, Lister JA, Main CJ. 1981. Identification of features associated with flying phobia in aircrew. Br J Psychiatry 139: 38–42. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
