Zoonotic Risk, Pathogenesis, and Transmission of Avian-Origin H3N2 Canine Influenza Virus
- PMID: 28814512
- PMCID: PMC5640866
- DOI: 10.1128/JVI.00637-17
Zoonotic Risk, Pathogenesis, and Transmission of Avian-Origin H3N2 Canine Influenza Virus
Erratum in
-
Erratum for Sun et al., "Zoonotic Risk, Pathogenesis, and Transmission of Avian-Origin H3N2 Canine Influenza Virus".J Virol. 2018 Mar 28;92(8):e02227-17. doi: 10.1128/JVI.02227-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29593063 Free PMC article. No abstract available.
Abstract
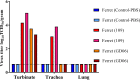
Two subtypes of influenza A virus (IAV), avian-origin canine influenza virus (CIV) H3N2 (CIV-H3N2) and equine-origin CIV H3N8 (CIV-H3N8), are enzootic in the canine population. Dogs have been demonstrated to seroconvert in response to diverse IAVs, and naturally occurring reassortants of CIV-H3N2 and the 2009 H1N1 pandemic virus (pdmH1N1) have been isolated. We conducted a thorough phenotypic evaluation of CIV-H3N2 in order to assess its threat to human health. Using ferret-generated antiserum, we determined that CIV-H3N2 is antigenically distinct from contemporary human H3N2 IAVs, suggesting that there may be minimal herd immunity in humans. We assessed the public health risk of CIV-H3N2 × pandemic H1N1 (pdmH1N1) reassortants by characterizing their in vitro genetic compatibility and in vivo pathogenicity and transmissibility. Using a luciferase minigenome assay, we quantified the polymerase activity of all possible 16 ribonucleoprotein (RNP) complexes (PB2, PB1, PA, NP) between CIV-H3N2 and pdmH1N1, identifying some combinations that were more active than either parental virus complex. Using reverse genetics and fixing the CIV-H3N2 hemagglutinin (HA), we found that 51 of the 127 possible reassortant viruses were viable and able to be rescued. Nineteen of these reassortant viruses had high-growth phenotypes in vitro, and 13 of these replicated in mouse lungs. A single reassortant with the NP and HA gene segments from CIV-H3N2 was selected for characterization in ferrets. The reassortant was efficiently transmitted by contact but not by the airborne route and was pathogenic in ferrets. Our results suggest that CIV-H3N2 reassortants may pose a moderate risk to public health and that the canine host should be monitored for emerging IAVs.IMPORTANCE IAV pandemics are caused by the introduction of novel viruses that are capable of efficient and sustained transmission into a human population with limited herd immunity. Dogs are a a potential mixing vessel for avian and mammalian IAVs and represent a human health concern due to their susceptibility to infection, large global population, and close physical contact with humans. Our results suggest that humans are likely to have limited preexisting immunity to CIV-H3N2 and that CIV-H3N2 × pdmH1N1 reassortants have moderate genetic compatibility and are transmissible by direct contact in ferrets. Our study contributes to the increasing evidence that surveillance of the canine population for IAVs is an important component of pandemic preparedness.
Keywords: 2009 H1N1 influenza A virus; A(H1N1)pdm09; H3N2; aerosol transmission; canine influenza virus; influenza A virus; reassortment; risk assessment; viral pathogenesis; zoonosis.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Mahy B. 1997. Influenza A virus (FLUA), p 170–171. Academic Press, San Diego, CA.
-
- Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RA, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theambooniers A, Tantilertcharoen R, Pattanarangsan R, Arya N, Ratanakorn P, Osterhaus DM, Poovorawan Y. 2004. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis 10:2189–2191. doi:10.3201/eid1012.040759. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

