Irgm1 coordinately regulates autoimmunity and host defense at select mucosal surfaces
- PMID: 28814662
- PMCID: PMC5621910
- DOI: 10.1172/jci.insight.91914
Irgm1 coordinately regulates autoimmunity and host defense at select mucosal surfaces
Abstract
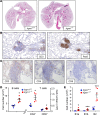
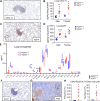
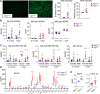
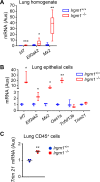
The pathogenesis of primary Sjogren's syndrome (SS), an autoimmune disease that targets the mucosa of exocrine tissues, is poorly understood. Although several mouse models have been developed that display features of SS, most of these are within the larger context of a lupus-like presentation. Immunity-related GTPase family M protein 1 (Irgm1) is an interferon-inducible cytoplasmic GTPase that is reported to regulate autophagy and mitochondrial homeostasis. Here, we report that naive Irgm1-/- mice display lymphocytic infiltration of multiple mucosal tissues including the lung in a manner reminiscent of SS, together with IgA class-predominant autoantibodies including anti-Ro and anti-La. This phenotype persists in the germ-free state, but is abolished by deletion of Irgm3. Irgm1-/- mice have increased local production in the lung of TECP15-idiotype IgA, a natural antibody with dual reactivity against host and pneumococcal phosphorylcholine. Associated with this, Irgm1-/- mice display enhanced opsonization and clearance of Streptococcus pneumoniae from the lung and increased survival from pneumococcal pneumonia. Taken together, our results identify Irgm1 as a master regulator of mucosal immunity that dually modulates evolutionarily conserved self- and other-directed immune responses at the interface of host with environment.
Keywords: Autoimmunity; Pulmonology.
Conflict of interest statement
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

