Rapamycin reversal of VEGF-C-driven lymphatic anomalies in the respiratory tract
- PMID: 28814666
- PMCID: PMC5621869
- DOI: 10.1172/jci.insight.90103
Rapamycin reversal of VEGF-C-driven lymphatic anomalies in the respiratory tract
Abstract
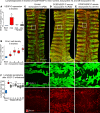
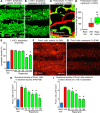
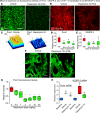
Lymphatic malformations are serious but poorly understood conditions that present therapeutic challenges. The goal of this study was to compare strategies for inducing regression of abnormal lymphatics and explore underlying mechanisms. CCSP-rtTA/tetO-VEGF-C mice, in which doxycycline regulates VEGF-C expression in the airway epithelium, were used as a model of pulmonary lymphangiectasia. After doxycycline was stopped, VEGF-C expression returned to normal, but lymphangiectasia persisted for at least 9 months. Inhibition of VEGFR-2/VEGFR-3 signaling, Notch, β-adrenergic receptors, or autophagy and antiinflammatory steroids had no noticeable effect on the amount or severity of lymphangiectasia. However, rapamycin inhibition of mTOR reduced lymphangiectasia by 76% within 7 days without affecting normal lymphatics. Efficacy of rapamycin was not increased by coadministration with the other agents. In prevention trials, rapamycin suppressed VEGF-C-driven mTOR phosphorylation and lymphatic endothelial cell sprouting and proliferation. However, in reversal trials, no lymphatic endothelial cell proliferation was present to block in established lymphangiectasia, and rapamycin did not increase caspase-dependent apoptosis. However, rapamycin potently suppressed Prox1 and VEGFR-3. These experiments revealed that lymphangiectasia is remarkably resistant to regression but is responsive to rapamycin, which rapidly reduces and normalizes the abnormal lymphatics without affecting normal lymphatics.
Keywords: Vascular Biology.
Conflict of interest statement
Figures





References
-
- Virchow R. Gesammelte Abhandlungen zur wissenschaftlichen Medicin. Frankfurt-am-Main, Germany: Verlag Meidinger Sohn & Comp.; 1856;983. https://archive.org/details/b21462161 Accessed July 18, 2017.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

