MTG16 is a tumor suppressor in colitis-associated carcinoma
- PMID: 28814670
- PMCID: PMC5621889
- DOI: 10.1172/jci.insight.78210
MTG16 is a tumor suppressor in colitis-associated carcinoma
Abstract
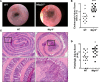
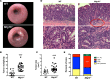
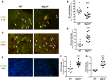
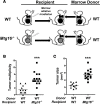
MTG16 is a member of the myeloid translocation gene (MTG) family of transcriptional corepressors. While MTGs were originally identified in chromosomal translocations in acute myeloid leukemia, recent studies have uncovered a role in intestinal biology. For example, Mtg16-/- mice have increased intestinal proliferation and are more sensitive to intestinal injury in colitis models. MTG16 is also underexpressed in patients with moderate/severe ulcerative colitis. Based on these findings, we postulated that MTG16 might protect against colitis-associated carcinogenesis. MTG16 was downregulated at the protein and RNA levels in patients with inflammatory bowel disease and in those with colitis-associated carcinoma. Mtg16-/- mice subjected to inflammatory carcinogenesis modeling exhibited worse colitis and increased tumor multiplicity and size. Loss of MTG16 also increased severity of dysplasia, apoptosis, proliferation, DNA damage, and WNT signaling. Moreover, transplantation of WT marrow into Mtg16-/- mice failed to rescue the Mtg16-/- protumorigenic phenotypes, indicating an epithelium-specific role for MTG16. While MTG dysfunction is widely appreciated in hematopoietic malignancies, the role of this gene family in epithelial homeostasis, and in colon cancer, was unrealized. This report identifies MTG16 as an important modulator of colitis and tumor development in inflammatory carcinogenesis.
Keywords: Gastroenterology; Genetics; Inflammation; Oncology.
Conflict of interest statement
Figures





Similar articles
-
MTG16 contributes to colonic epithelial integrity in experimental colitis.Gut. 2013 Oct;62(10):1446-55. doi: 10.1136/gutjnl-2011-301439. Epub 2012 Jul 24. Gut. 2013. PMID: 22833394 Free PMC article.
-
Kaiso is required for MTG16-dependent effects on colitis-associated carcinoma.Oncogene. 2019 Jun;38(25):5091-5106. doi: 10.1038/s41388-019-0777-7. Epub 2019 Mar 11. Oncogene. 2019. PMID: 30858547 Free PMC article.
-
Myeloid translocation genes differentially regulate colorectal cancer programs.Oncogene. 2016 Dec 8;35(49):6341-6349. doi: 10.1038/onc.2016.167. Epub 2016 Jun 6. Oncogene. 2016. PMID: 27270437 Free PMC article.
-
Emerging Roles of MTG16 in Cell-Fate Control of Hematopoietic Stem Cells and Cancer.Stem Cells Int. 2017;2017:6301385. doi: 10.1155/2017/6301385. Epub 2017 Nov 22. Stem Cells Int. 2017. PMID: 29358956 Free PMC article. Review.
-
Chronic inflammatory bowel disease and cancer.Hepatogastroenterology. 2000 Jan-Feb;47(31):57-70. Hepatogastroenterology. 2000. PMID: 10690586 Review.
Cited by
-
MTGR1 is required to maintain small intestinal stem cell populations.Cell Death Differ. 2024 Sep;31(9):1170-1183. doi: 10.1038/s41418-024-01346-x. Epub 2024 Jul 25. Cell Death Differ. 2024. PMID: 39048708 Free PMC article.
-
Mtg16 NHR1 mutations cause defects in lymphopoiesis and the response to anemia.Exp Hematol. 2025 Jul;147:104793. doi: 10.1016/j.exphem.2025.104793. Epub 2025 Apr 30. Exp Hematol. 2025. PMID: 40316246
-
The Transcription Co-Repressors MTG8 and MTG16 Regulate Exit of Intestinal Stem Cells From Their Niche and Differentiation Into Enterocyte vs Secretory Lineages.Gastroenterology. 2020 Oct;159(4):1328-1341.e3. doi: 10.1053/j.gastro.2020.06.012. Epub 2020 Jun 15. Gastroenterology. 2020. PMID: 32553763 Free PMC article.
-
MTG16 regulates colonic epithelial differentiation, colitis, and tumorigenesis by repressing E protein transcription factors.JCI Insight. 2022 May 23;7(10):e153045. doi: 10.1172/jci.insight.153045. JCI Insight. 2022. PMID: 35503250 Free PMC article.
References
-
- Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;373(2):195. - PubMed
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- T32 DK007673/DK/NIDDK NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- R01 DK099204/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 AT004821/AT/NCCIH NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- I01 BX001426/BX/BLRD VA/United States
- F32 DK108492/DK/NIDDK NIH HHS/United States
- K23 DK094832/DK/NIDDK NIH HHS/United States
- S10 OD021630/OD/NIH HHS/United States
- K08 DK080221/DK/NIDDK NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- I01 BX001453/BX/BLRD VA/United States
- IK2 BX002126/BX/BLRD VA/United States
- F31 CA167920/CA/NCI NIH HHS/United States
- F30 DK096718/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

