VIPAR, a quantitative approach to 3D histopathology applied to lymphatic malformations
- PMID: 28814672
- PMCID: PMC5621876
- DOI: 10.1172/jci.insight.93424
VIPAR, a quantitative approach to 3D histopathology applied to lymphatic malformations
Abstract
Background: Lack of investigatory and diagnostic tools has been a major contributing factor to the failure to mechanistically understand lymphedema and other lymphatic disorders in order to develop effective drug and surgical therapies. One difficulty has been understanding the true changes in lymph vessel pathology from standard 2D tissue sections.
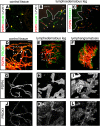
Methods: VIPAR (volume information-based histopathological analysis by 3D reconstruction and data extraction), a light-sheet microscopy-based approach for the analysis of tissue biopsies, is based on digital reconstruction and visualization of microscopic image stacks. VIPAR allows semiautomated segmentation of the vasculature and subsequent nonbiased extraction of characteristic vessel shape and connectivity parameters. We applied VIPAR to analyze biopsies from healthy lymphedematous and lymphangiomatous skin.
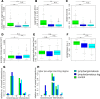
Results: Digital 3D reconstruction provided a directly visually interpretable, comprehensive representation of the lymphatic and blood vessels in the analyzed tissue volumes. The most conspicuous features were disrupted lymphatic vessels in lymphedematous skin and a hyperplasia (4.36-fold lymphatic vessel volume increase) in the lymphangiomatous skin. Both abnormalities were detected by the connectivity analysis based on extracted vessel shape and structure data. The quantitative evaluation of extracted data revealed a significant reduction of lymphatic segment length (51.3% and 54.2%) and straightness (89.2% and 83.7%) for lymphedematous and lymphangiomatous skin, respectively. Blood vessel length was significantly increased in the lymphangiomatous sample (239.3%).
Conclusion: VIPAR is a volume-based tissue reconstruction data extraction and analysis approach that successfully distinguished healthy from lymphedematous and lymphangiomatous skin. Its application is not limited to the vascular systems or skin.
Funding: Max Planck Society, DFG (SFB 656), and Cells-in-Motion Cluster of Excellence EXC 1003.
Keywords: Dermatology; Vascular Biology.
Conflict of interest statement
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

