In silico investigation of a KCNQ1 mutation associated with short QT syndrome
- PMID: 28814790
- PMCID: PMC5559555
- DOI: 10.1038/s41598-017-08367-2
In silico investigation of a KCNQ1 mutation associated with short QT syndrome
Abstract
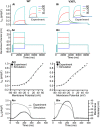
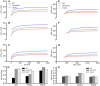
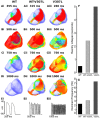
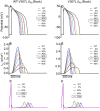
Short QT syndrome (SQTS) is a rare condition characterized by abnormally 'short' QT intervals on the ECG and increased susceptibility to cardiac arrhythmias and sudden death. This simulation study investigated arrhythmia dynamics in multi-scale human ventricle models associated with the SQT2-related V307L KCNQ1 'gain-of-function' mutation, which increases slow-delayed rectifier potassium current (IKs). A Markov chain (MC) model recapitulating wild type (WT) and V307L mutant IKs kinetics was incorporated into a model of the human ventricular action potential (AP) for investigation of QT interval changes and arrhythmia substrates. In addition, the degree of simulated IKs inhibition necessary to normalize the QT interval and terminate re-entry in SQT2 conditions was quantified. The developed MC model accurately reproduced AP shortening and reduced effective refractory period associated with altered IKs kinetics in homozygous (V307L) and heterozygous (WT-V307L) mutation conditions, which increased the lifespan and dominant frequency of re-entry in 3D human ventricle models. IKs reductions of 58% and 65% were sufficient to terminate re-entry in WT-V307L and V307L conditions, respectively. This study further substantiates a causal link between the V307L KCNQ1 mutation and pro-arrhythmia in human ventricles, and establishes partial inhibition of IKs as a potential anti-arrhythmic strategy in SQT2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

