Exploring the long-term safety of asenapine in adults with schizophrenia in a double-blind, fixed-dose, extension study
- PMID: 28814871
- PMCID: PMC5546824
- DOI: 10.2147/NDT.S130211
Exploring the long-term safety of asenapine in adults with schizophrenia in a double-blind, fixed-dose, extension study
Abstract
Purpose: The primary objective of this study was to assess long-term safety with sublingual asenapine 2.5 or 5 mg twice daily (BID) in patients with schizophrenia.
Patients and methods: Actively treated patients on asenapine 2.5 mg BID, asenapine 5 mg BID, or olanzapine 15 mg once daily (QD) who completed a 6-week randomized, double-blind, placebo- and olanzapine-controlled study continued lead-in treatment in this 26-week, multicenter, double-blind, double-dummy, olanzapine-controlled Phase IIIB extension study; placebo patients were assigned to asenapine 2.5 mg BID treatment. Safety analyses were based on the all treated set (patients who received one or more doses of extension trial medication); change from baseline analyses used the acute study baseline. Treatment-emergent adverse events (TEAEs) and changes in laboratory parameters were monitored; weight change for asenapine versus olanzapine was the key secondary objective. Descriptive statistics were used; weight change was analyzed using a mixed-model repeated-measure approach.
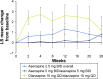
Results: Of the 120 patients in the all-treated set, 60% completed treatment (asenapine 2.5 mg BID 66.1% overall, asenapine 5 mg BID 52.4%, olanzapine 15 mg QD 56.3%). The incidence of TEAEs was higher for placebo patients from the lead-in study who switched to asenapine 2.5 mg BID for extension treatment (71.0%) versus patients continuing asenapine 2.5 mg BID (38.7%), asenapine 5 mg BID (38.1%), or olanzapine 15 mg QD (25.0%). The most common TEAE (≥5% in every group) was worsening of schizophrenia. Least squares mean change in body weight from the acute study baseline to week 26 was +0.6 kg for overall asenapine 2.5 mg BID, +0.8 kg for asenapine 5 mg BID, and +1.2 kg for olanzapine 15 mg QD. There were no clinically relevant changes in metabolic parameters; values were generally similar across treatment groups.
Conclusion: Asenapine 2.5 mg BID and 5 mg BID were generally well tolerated in long-term treatment. Weight gain was less for overall asenapine 2.5 mg BID and 5 mg BID than for olanzapine 15 mg QD.
Keywords: asenapine; long-term; olanzapine; safety; schizophrenia; weight.
Conflict of interest statement
Disclosure S Durgam and X Wu acknowledge a potential conflict of interest as employees of Allergan. RP Landbloom and M Mackle acknowledge a potential conflict of interest as employees of Merck. At the time of the study, M Mathews was employed by Forest Research Institute (now Allergan). HA Nasrallah has been a consultant for Acadia, Alkermes, Allergan, Boehringer Ingelheim, Grünenthal USA, Janssen Pharmaceuticals, Lundbeck, Merck Sharp and Dohme, Novartis, Otsuka Pharmaceutical, Roche/Genentech, Sunovion Pharmaceuticals, and Vanda Pharmaceuticals; he has served on speakers’ bureaus for Acadia, Alkermes, Allergan, Janssen Pharmaceuticals, Lundbeck, Merck Sharpe and Dohme, Otsuka Pharmaceuticals, Sunovion Pharmaceuticals, and Vanda; and he has received grant/research support from Forest Pharmaceuticals, Otsuka Pharmaceutical, and Roche/Genentech. The authors report no other conflicts of interest in this work.
Figures


References
-
- Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183–194. - PubMed
-
- Palmer BA, Pankratz V, Bostwick J. The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. 2005;62(3):247–253. - PubMed
-
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4: clinical features and conceptualization. Schizophr Res. 2009;110(1–3):1–23. - PubMed
-
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

