The effect of lipiodol deposition in HCC after TACE on the necrosis range of PMCT
- PMID: 28814882
- PMCID: PMC5546818
- DOI: 10.2147/OTT.S137312
The effect of lipiodol deposition in HCC after TACE on the necrosis range of PMCT
Abstract
Objective: To study the impact of lipiodol deposition in the lesion of hepatocellular carcinoma (HCC) after transarterial chemoembolization (TACE) on the necrosis area of percutaneous microwave coagulation therapy (PMCT).
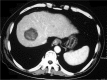
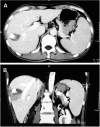
Materials and methods: A total of 44 patients with HCC with 56 nodules, with a size ranging from 1.5 to 3.5 cm, was selected in our study. About 23 patients (26 nodules) underwent PMCT treatment only as Group A and 21 patients (30 nodules) were treated by PMCT-combined TACE as Group B. All patients underwent PMCT with single-electrode and one-point ablation. Paired t-test was used to analyze pre- and postoperatively the volume of tumor and the necrosis volume after PMCT. Independent t-test was used to compare the difference in the necrosis area between two groups (α=0.05).
Results: All patients underwent PMCT or PMCT combined with TACE successfully. The tumor and necrosis size of Group A was 16.29±19.23 cm3 and 17.98±18.49 cm3 (P=0.650), and 11.95±12.78 cm3 and 16.60±11.70 cm3 of Group B (P=0.017). There was no significant difference on necrosis volume between the two groups (P=0.581). The necrosis area of Group B was larger than the size of the tumor (P=0.017), but the ablation area of the two groups was smaller than the theoretic area (P=0.001). (The theoretic area means that the necrosis area of ablation should be 1.0 cm larger than the tumor in diameter.).
Conclusion: PMCT combined with TACE could enlarge the ablation area, but will not lead to an ideal necrosis area than the PMCT alone. The lipiodol deposition in the tumor lesion may hinder the expansion of the heating field. Therefore, further research was needed.
Keywords: hepatocellular carcinoma; lipiodol; percutaneous microwave coagulation therapy; transarterial chemoembolization.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy.J Gastroenterol Hepatol. 2013 Mar;28(3):456-63. doi: 10.1111/jgh.12088. J Gastroenterol Hepatol. 2013. PMID: 23216261
-
Combined treatment, TACE and RF ablation, in HCC: preliminary results.Radiol Med. 2002 Nov-Dec;104(5-6):412-20. Radiol Med. 2002. PMID: 12589262 English, Italian.
-
Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas.J Gastroenterol Hepatol. 2009 Feb;24(2):223-7. doi: 10.1111/j.1440-1746.2008.05596.x. Epub 2008 Sep 24. J Gastroenterol Hepatol. 2009. PMID: 18823439
-
The efficacy of transarterial chemoembolization combined with microwave ablation for unresectable hepatocellular carcinoma: a systematic review and meta-analysis.Int J Hyperthermia. 2019;36(1):1288-1296. doi: 10.1080/02656736.2019.1692148. Int J Hyperthermia. 2019. PMID: 31852267
-
The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma.Anal Cell Pathol (Amst). 2019 Aug 26;2019:8619096. doi: 10.1155/2019/8619096. eCollection 2019. Anal Cell Pathol (Amst). 2019. PMID: 31534899 Free PMC article. Review.
Cited by
-
Transcatheter arterial chemoembolization monotherapy vs combined transcatheter arterial chemoembolization-percutaneous microwave coagulation therapy for massive hepatocellular carcinoma (≥10 cm).Cancer Manag Res. 2018 Nov 1;10:5273-5282. doi: 10.2147/CMAR.S172395. eCollection 2018. Cancer Manag Res. 2018. PMID: 30464624 Free PMC article.
-
Comparison of clinical outcomes of laparoscopic versus open surgery for recurrent hepatocellular carcinoma: a meta-analysis.Surg Endosc. 2019 Nov;33(11):3550-3557. doi: 10.1007/s00464-019-06996-4. Epub 2019 Jul 24. Surg Endosc. 2019. PMID: 31342257
-
A Pretreatment CT Model Predicts Survival Following Chemolipiodolization in Patients With Hepatocellular Carcinoma.Technol Cancer Res Treat. 2019 Jan 1;18:1533033819844488. doi: 10.1177/1533033819844488. Technol Cancer Res Treat. 2019. PMID: 31204599 Free PMC article.
-
Nurses' comfort care of transarterial chemoembolization patients based on their perceptions around postembolization syndrome and symptom interference.Nurs Open. 2023 May;10(5):2877-2885. doi: 10.1002/nop2.1529. Epub 2022 Dec 24. Nurs Open. 2023. PMID: 36565057 Free PMC article.
-
Clinical Efficacy of Transcatheter Arterial Chemoembolization Combined With Percutaneous Microwave Coagulation Therapy for Advanced Hepatocellular Carcinoma.Gastroenterology Res. 2024 Aug;17(4):175-182. doi: 10.14740/gr1713. Epub 2024 Jul 18. Gastroenterology Res. 2024. PMID: 39247707 Free PMC article.
References
-
- Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. - PubMed
-
- Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29(6):558–568. - PubMed
-
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103(6):1201–1209. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

