Clinical Trials.Gov: A Topical Analyses
- PMID: 28815102
- PMCID: PMC5543348
Clinical Trials.Gov: A Topical Analyses
Abstract
ClinicalTrials.gov was established as a web-based registry for clinical trials of human participants in 2000. Mandatory registration started in 2008. Given more than a decade of registered trials, it's important to understand the "topic" areas and their evolution over time from this resource. This information may help in identifying current knowledge gaps. We use dynamic topic model (DTM) methods to discover topics and their evolution over last 17 years. Our model suggests that there are disease or organ specific trials such as 'Cardiovascular disorders', Heart & Brain conditions', or 'Breast & Prostate cancer' as well as trials registered for general health. General health trials are less likely to be FDA regulated, but both health and pain management, as well as surgical, heart, and brain trials have upward trend in recent years while advanced cancer trials have downward trended. Our model derives unique insights from metadata associated with each topic area.
Figures




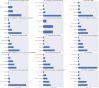
References
-
- Zarin DA, Tse T, Williams RJ, Carr S. Trial Reporting in ClinicalTrials.gov - The Final Rule. N Engl J Med. 2016 Sep 16; - PMC - PubMed
-
- Cepeda MS, Lobanov V, Berlin JA. From ClinicalTrials.gov trial registry to an analysis-ready database of clinical trial results. Clin Trials Lond Engl. 2013 Apr;10(2):347–8. - PubMed
-
- Holman L, Head ML, Lanfear R, Jennions MD. Evidence of Experimental Bias in the Life Sciences: Why We Need Blind Data Recording. PLoS Biol [Internet] 2015 Jul 8;13(7) [cited 2016 Sep 21]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4496034/ - PMC - PubMed
LinkOut - more resources
Full Text Sources
