Evolving Research Data Sharing Networks to Clinical App Sharing Networks
- PMID: 28815145
- PMCID: PMC5543356
Evolving Research Data Sharing Networks to Clinical App Sharing Networks
Abstract
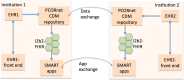
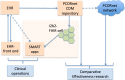
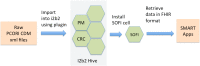
Research networks for data sharing are growing into a large platform for pragmatic clinical trials to generate quality evidence for shared medical decision-making. Institutions partnering in the networks have made large investments in developing the infrastructure for sharing data. We investigate whether institutions partnering on Patient-Centered Outcomes Research Institute's (PCORI) network can share clinical apps. At two different sites, we imported patient data in PCORI's clinical data model (CDM) format into i2b2 repositories, and adapted the SMART-on-FHIR cell to perform CDM-to-FHIR translation, serving demographics, laboratory results and diagnoses. We performed manual validations and tested the platform using four apps from the SMART app gallery. Our study demonstrates an approach to extend the research infrastructure to allow the partnering institutions to run shared clinical apps, and highlights the involved challenges. Our results, tooling and publically accessible data service can potentially transform research networks into clinical app sharing networks and pave the way towards a learning health system.
Figures
References
-
- Gabriel S.E., Normand S.L. N Engl J Med. Getting the methods right--the foundation of patient-centered outcomes research. 2012;367(9):787–90. - PubMed
-
- Lauer M.S, D'Agostino R.B., Sr. N Engl J Med. The randomized registry trial--the next disruptive technology in clinical research? 2013;369(17):1579–81. - PubMed
-
- Psaty B.M., Breckenridge A.M. Mini-Sentinel and Regulatory Science — Big Data Rendered Fit and Functional. The New England Journal of Medicine. 2014;370(23):2165–2167. - PubMed
-
- Standards and Interoperability framework. 2013. http://wiki.siframework.org/ [cited 2013 1 March]; Available from.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
