Inhibition of γ-Secretase Leads to an Increase in Presenilin-1
- PMID: 28815510
- PMCID: PMC5948247
- DOI: 10.1007/s12035-017-0705-1
Inhibition of γ-Secretase Leads to an Increase in Presenilin-1
Abstract
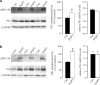
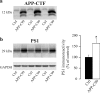
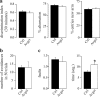
γ-Secretase inhibitors (GSIs) are potential therapeutic agents for Alzheimer's disease (AD); however, trials have proven disappointing. We addressed the possibility that γ-secretase inhibition can provoke a rebound effect, elevating the levels of the catalytic γ-secretase subunit, presenilin-1 (PS1). Acute treatment of SH-SY5Y cells with the GSI LY-374973 (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester, DAPT) augments PS1, in parallel with increases in other γ-secretase subunits nicastrin, presenilin enhancer 2, and anterior pharynx-defective 1, yet with no increase in messenger RNA expression. Over-expression of the C-terminal fragment (CTF) of APP, C99, also triggered an increase in PS1. Similar increases in PS1 were evident in primary neurons treated repeatedly (4 days) with DAPT or with the GSI BMS-708163 (avagacestat). Likewise, rats examined after 21 days administered with avagacestat (40 mg/kg/day) had more brain PS1. Sustained γ-secretase inhibition did not exert a long-term effect on PS1 activity, evident through the decrease in CTFs of APP and ApoER2. Prolonged avagacestat treatment of rats produced a subtle impairment in anxiety-like behavior. The rebound increase in PS1 in response to GSIs must be taken into consideration for future drug development.
Keywords: Alzheimer’s disease; Presenilin-1; Therapy; γ-Secretase inhibitor.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no competing interests.
Figures



Similar articles
-
Specificity of presenilin-1- and presenilin-2-dependent γ-secretases towards substrate processing.J Cell Mol Med. 2018 Feb;22(2):823-833. doi: 10.1111/jcmm.13364. Epub 2017 Oct 10. J Cell Mol Med. 2018. PMID: 28994238 Free PMC article.
-
Identification of presenilin 1-selective γ-secretase inhibitors with reconstituted γ-secretase complexes.Biochemistry. 2011 Jun 7;50(22):4973-80. doi: 10.1021/bi200026m. Epub 2011 May 13. Biochemistry. 2011. PMID: 21528914
-
Identification of gamma-secretase inhibitor potency determinants on presenilin.J Biol Chem. 2008 Feb 1;283(5):2927-38. doi: 10.1074/jbc.M708870200. Epub 2007 Nov 21. J Biol Chem. 2008. PMID: 18032377
-
Amyloidogenic and anti-amyloidogenic properties of presenilin 1.Adv Pharmacol. 2021;90:239-251. doi: 10.1016/bs.apha.2020.09.010. Epub 2020 Oct 24. Adv Pharmacol. 2021. PMID: 33706935 Review.
-
Recent progress in the medicinal chemistry of gamma-secretase inhibitors.Curr Top Med Chem. 2008;8(1):17-33. doi: 10.2174/156802608783334088. Curr Top Med Chem. 2008. PMID: 18220929 Review.
Cited by
-
Characterization of Cerebrospinal Fluid BACE1 Species.Mol Neurobiol. 2019 Dec;56(12):8603-8616. doi: 10.1007/s12035-019-01677-8. Epub 2019 Jul 9. Mol Neurobiol. 2019. PMID: 31290061
-
Natural bioactive compounds as notch signaling modulators: cutting-edge strategies for cancer therapy.Med Oncol. 2025 Jul 22;42(8):363. doi: 10.1007/s12032-025-02792-4. Med Oncol. 2025. PMID: 40694161 Review.
-
Amyloid precursor protein glycosylation is altered in the brain of patients with Alzheimer's disease.Alzheimers Res Ther. 2020 Aug 12;12(1):96. doi: 10.1186/s13195-020-00664-9. Alzheimers Res Ther. 2020. PMID: 32787955 Free PMC article.
-
Role of Presenilin-1 in Aggressive Human Melanoma.Int J Mol Sci. 2022 Apr 28;23(9):4904. doi: 10.3390/ijms23094904. Int J Mol Sci. 2022. PMID: 35563300 Free PMC article.
-
Presenilin Is Essential for ApoE Secretion, a Novel Role of Presenilin Involved in Alzheimer's Disease Pathogenesis.J Neurosci. 2022 Feb 23;42(8):1574-1586. doi: 10.1523/JNEUROSCI.2039-21.2021. Epub 2022 Jan 5. J Neurosci. 2022. PMID: 34987110 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

